Abstract
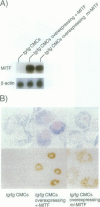
Mast cells develop when spleen cells of mice are cultured in the medium containing interleukin (IL)-3. Cultured mast cells (CMCs) show apoptosis when they are incubated in the medium without IL-3. We obtained CMCs from tg/tg mice that did not express the transcription factor encoded by the mi gene (MITF) due to the integration of a transgene at its 5' flanking region. MITF is a member of the basic-helix-loop-helix-leucine zipper (bHLH-Zip) protein family of transcription factors. We investigated the effect of MITF on the apoptosis of CMCs after removal of IL-3. When cDNA encoding normal MITF ((+)-MITF) was introduced into tg/tg CMCs with the retroviral vector, the apoptosis of tg/tg CMCs was significantly accelerated. The mutant mi allele represents a deletion of an arginine at the basic domain of MITF. The apoptosis of tg/tg CMCs was not accelerated by the introduction of cDNA encoding mi-MITF. The overexpression of (+)-MITF was not prerequisite to the acceleration of the apoptosis, as the apoptotic process proceeded faster in +/+ CMCs than in mi/mi CMCs. The Ba/F3 lymphoid cell line is also dependent on IL-3, and Ba/F3 cells show apoptosis after removal of IL-3. The c-myc gene encodes another transcription factor of the bHLH-Zip family, and the overexpression of the c-myc gene accelerated the apoptosis of Ba/F3 cells. However, the overexpression of (+)-MITF did not accelerate the apoptosis of Ba/F3 cells. The (+)-MITF appeared to play some roles for the acceleration of the apoptosis specifically in the mast cell lineage.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bello-Fernandez C., Packham G., Cleveland J. L. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7804–7808. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley N. J., Eisen T., Goding C. R. Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol. 1994 Dec;14(12):7996–8006. doi: 10.1128/mcb.14.12.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenisty N., Leder A., Kuo A., Leder P. An embryonically expressed gene is a target for c-Myc regulation via the c-Myc-binding sequence. Genes Dev. 1992 Dec;6(12B):2513–2523. doi: 10.1101/gad.6.12b.2513. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Ebi Y., Kanakura Y., Jippo-Kanemoto T., Tsujimura T., Furitsu T., Ikeda H., Adachi S., Kasugai T., Nomura S., Kanayama Y. Low c-kit expression of cultured mast cells of mi/mi genotype may be involved in their defective responses to fibroblasts that express the ligand for c-kit. Blood. 1992 Sep 15;80(6):1454–1462. [PubMed] [Google Scholar]
- Ebi Y., Kasugai T., Seino Y., Onoue H., Kanemoto T., Kitamura Y. Mechanism of mast cell deficiency in mutant mice of mi/mi genotype: an analysis by co-culture of mast cells and fibroblasts. Blood. 1990 Mar 15;75(6):1247–1251. [PubMed] [Google Scholar]
- Eilers M., Schirm S., Bishop J. M. The MYC protein activates transcription of the alpha-prothymosin gene. EMBO J. 1991 Jan;10(1):133–141. doi: 10.1002/j.1460-2075.1991.tb07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck L. Berberine sulphate binding to mast cell polyanions: a cytofluorometric method for the quantitation of heparin. Histochemistry. 1974;42(4):301–313. doi: 10.1007/BF00492678. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Galli S. J. New concepts about the mast cell. N Engl J Med. 1993 Jan 28;328(4):257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemesath T. J., Steingrímsson E., McGill G., Hansen M. J., Vaught J., Hodgkinson C. A., Arnheiter H., Copeland N. G., Jenkins N. A., Fisher D. E. microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 1994 Nov 15;8(22):2770–2780. doi: 10.1101/gad.8.22.2770. [DOI] [PubMed] [Google Scholar]
- Hodgkinson C. A., Moore K. J., Nakayama A., Steingrímsson E., Copeland N. G., Jenkins N. A., Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993 Jul 30;74(2):395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Hughes D. P., Crispe I. N. A naturally occurring soluble isoform of murine Fas generated by alternative splicing. J Exp Med. 1995 Nov 1;182(5):1395–1401. doi: 10.1084/jem.182.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M. J., Lingrel J. B., Krakowsky J. M., Anderson K. P. A helix-loop-helix transcription factor-like gene is located at the mi locus. J Biol Chem. 1993 Oct 5;268(28):20687–20690. [PubMed] [Google Scholar]
- Iemura A., Tsai M., Ando A., Wershil B. K., Galli S. J. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am J Pathol. 1994 Feb;144(2):321–328. [PMC free article] [PubMed] [Google Scholar]
- Isozaki K., Tsujimura T., Nomura S., Morii E., Koshimizu U., Nishimune Y., Kitamura Y. Cell type-specific deficiency of c-kit gene expression in mutant mice of mi/mi genotype. Am J Pathol. 1994 Oct;145(4):827–836. [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Redmond S., Wade-Evans A. Cloning and expression analysis of full length mouse cDNA sequences encoding the transformation associated protein p53. Nucleic Acids Res. 1984 Jul 25;12(14):5609–5626. doi: 10.1093/nar/12.14.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jippo T., Tsujino K., Kim H. M., Kim D. K., Lee Y. M., Nawa Y., Kitamura Y. Expression of mast-cell-specific proteases in tissues of mice studied by in situ hybridization. Am J Pathol. 1997 Apr;150(4):1373–1382. [PMC free article] [PubMed] [Google Scholar]
- Kasugai T., Oguri K., Jippo-Kanemoto T., Morimoto M., Yamatodani A., Yoshida K., Ebi Y., Isozaki K., Tei H., Tsujimura T. Deficient differentiation of mast cells in the skin of mi/mi mice. Usefulness of in situ hybridization for evaluation of mast cell phenotype. Am J Pathol. 1993 Nov;143(5):1337–1347. [PMC free article] [PubMed] [Google Scholar]
- Kitayama H., Kanakura Y., Furitsu T., Tsujimura T., Oritani K., Ikeda H., Sugahara H., Mitsui H., Kanayama Y., Kitamura Y. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood. 1995 Feb 1;85(3):790–798. [PubMed] [Google Scholar]
- Kume T. U., Takada S., Obinata M. Probability that the commitment of murine erythroleukemia cell differentiation is determined by the c-myc level. J Mol Biol. 1988 Aug 20;202(4):779–786. doi: 10.1016/0022-2836(88)90558-x. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Malde P., Collins M. K. Disregulation of Myc expression in murine bone marrow cells results in an inability to proliferate in sub-optimal growth factor and an increased sensitivity to DNA damage. Int Immunol. 1994 Aug;6(8):1169–1176. doi: 10.1093/intimm/6.8.1169. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Mekori Y. A., Oh C. K., Metcalfe D. D. IL-3-dependent murine mast cells undergo apoptosis on removal of IL-3. Prevention of apoptosis by c-kit ligand. J Immunol. 1993 Oct 1;151(7):3775–3784. [PubMed] [Google Scholar]
- Morii E., Takebayashi K., Motohashi H., Yamamoto M., Nomura S., Kitamura Y. Loss of DNA binding ability of the transcription factor encoded by the mutant mi locus. Biochem Biophys Res Commun. 1994 Dec 15;205(2):1299–1304. doi: 10.1006/bbrc.1994.2806. [DOI] [PubMed] [Google Scholar]
- Morii E., Tsujimura T., Jippo T., Hashimoto K., Takebayashi K., Tsujino K., Nomura S., Yamamoto M., Kitamura Y. Regulation of mouse mast cell protease 6 gene expression by transcription factor encoded by the mi locus. Blood. 1996 Oct 1;88(7):2488–2494. [PubMed] [Google Scholar]
- Nakahata T., Spicer S. S., Cantey J. R., Ogawa M. Clonal assay of mouse mast cell colonies in methylcellulose culture. Blood. 1982 Aug;60(2):352–361. [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985 Sep 1;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini M., Silini E., Kozak C., Tsujimoto Y., Croce C. M. Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell. 1987 May 22;49(4):455–463. doi: 10.1016/0092-8674(87)90448-x. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Reisman D., Elkind N. B., Roy B., Beamon J., Rotter V. c-Myc trans-activates the p53 promoter through a required downstream CACGTG motif. Cell Growth Differ. 1993 Feb;4(2):57–65. [PubMed] [Google Scholar]
- Reynolds D. S., Stevens R. L., Gurley D. S., Lane W. S., Austen K. F., Serafin W. E. Isolation and molecular cloning of mast cell carboxypeptidase A. A novel member of the carboxypeptidase gene family. J Biol Chem. 1989 Nov 25;264(33):20094–20099. [PubMed] [Google Scholar]
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993 Mar;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Stechschulte D. J., Sharma R., Dileepan K. N., Simpson K. M., Aggarwal N., Clancy J., Jr, Jilka R. L. Effect of the mi allele on mast cells, basophils, natural killer cells, and osteoclasts in C57Bl/6J mice. J Cell Physiol. 1987 Sep;132(3):565–570. doi: 10.1002/jcp.1041320321. [DOI] [PubMed] [Google Scholar]
- Steingrímsson E., Moore K. J., Lamoreux M. L., Ferré-D'Amaré A. R., Burley S. K., Zimring D. C., Skow L. C., Hodgkinson C. A., Arnheiter H., Copeland N. G. Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nat Genet. 1994 Nov;8(3):256–263. doi: 10.1038/ng1194-256. [DOI] [PubMed] [Google Scholar]
- Stevens J., Loutit J. F. Mast cells in spotted mutant mice (W Ph mi). Proc R Soc Lond B Biol Sci. 1982 Jun 22;215(1200):405–409. doi: 10.1098/rspb.1982.0050. [DOI] [PubMed] [Google Scholar]
- Stewart B. W. Mechanisms of apoptosis: integration of genetic, biochemical, and cellular indicators. J Natl Cancer Inst. 1994 Sep 7;86(17):1286–1296. doi: 10.1093/jnci/86.17.1286. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Takebayashi K., Chida K., Tsukamoto I., Morii E., Munakata H., Arnheiter H., Kuroki T., Kitamura Y., Nomura S. The recessive phenotype displayed by a dominant negative microphthalmia-associated transcription factor mutant is a result of impaired nucleation potential. Mol Cell Biol. 1996 Mar;16(3):1203–1211. doi: 10.1128/mcb.16.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura T., Morii E., Nozaki M., Hashimoto K., Moriyama Y., Takebayashi K., Kondo T., Kanakura Y., Kitamura Y. Involvement of transcription factor encoded by the mi locus in the expression of c-kit receptor tyrosine kinase in cultured mast cells of mice. Blood. 1996 Aug 15;88(4):1225–1233. [PubMed] [Google Scholar]
- Västrik I., Mäkelä T. P., Koskinen P. J., Klefstrom J., Alitalo K. Myc protein: partners and antagonists. Crit Rev Oncog. 1994;5(1):59–68. doi: 10.1615/critrevoncog.v5.i1.30. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Itoh N., Yonehara S., Copeland N. G., Jenkins N. A., Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992 Feb 15;148(4):1274–1279. [PubMed] [Google Scholar]
- Yasumoto K., Yokoyama K., Shibata K., Tomita Y., Shibahara S. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol Cell Biol. 1994 Dec;14(12):8058–8070. doi: 10.1128/mcb.14.12.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee N. S., Paek I., Besmer P. Role of kit-ligand in proliferation and suppression of apoptosis in mast cells: basis for radiosensitivity of white spotting and steel mutant mice. J Exp Med. 1994 Jun 1;179(6):1777–1787. doi: 10.1084/jem.179.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]