Abstract
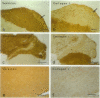
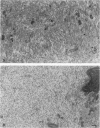
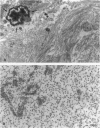
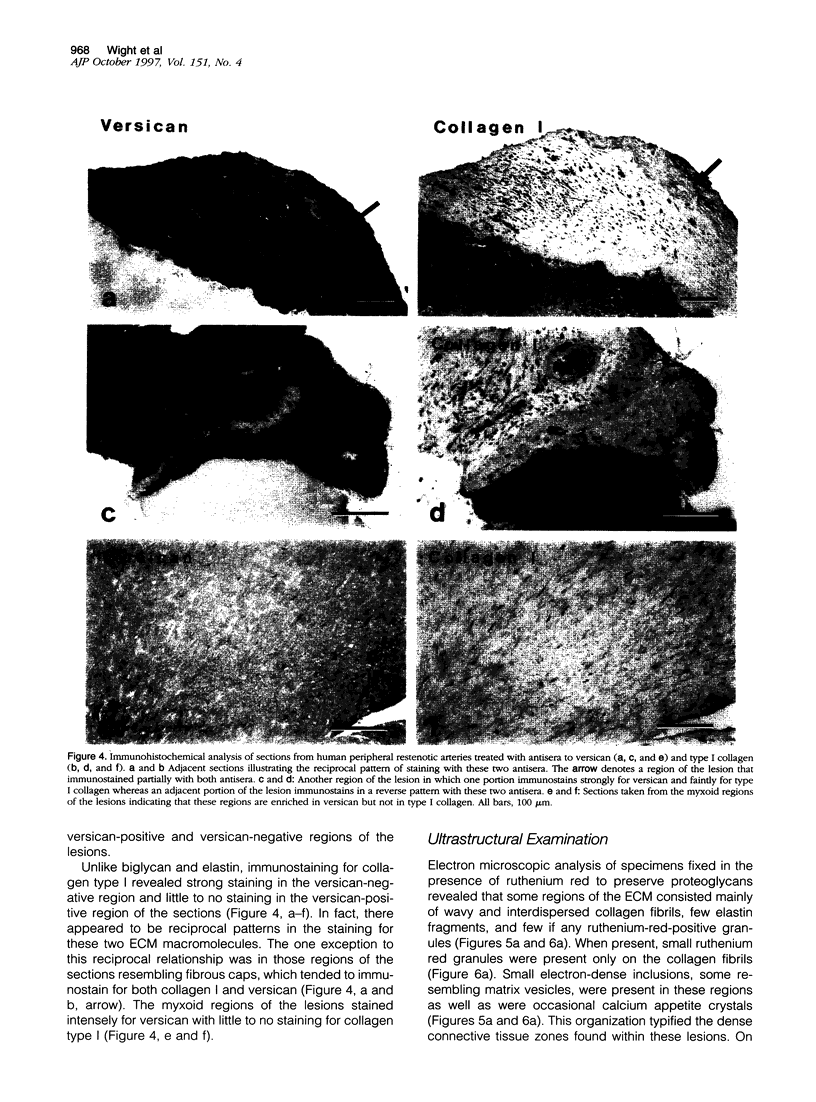
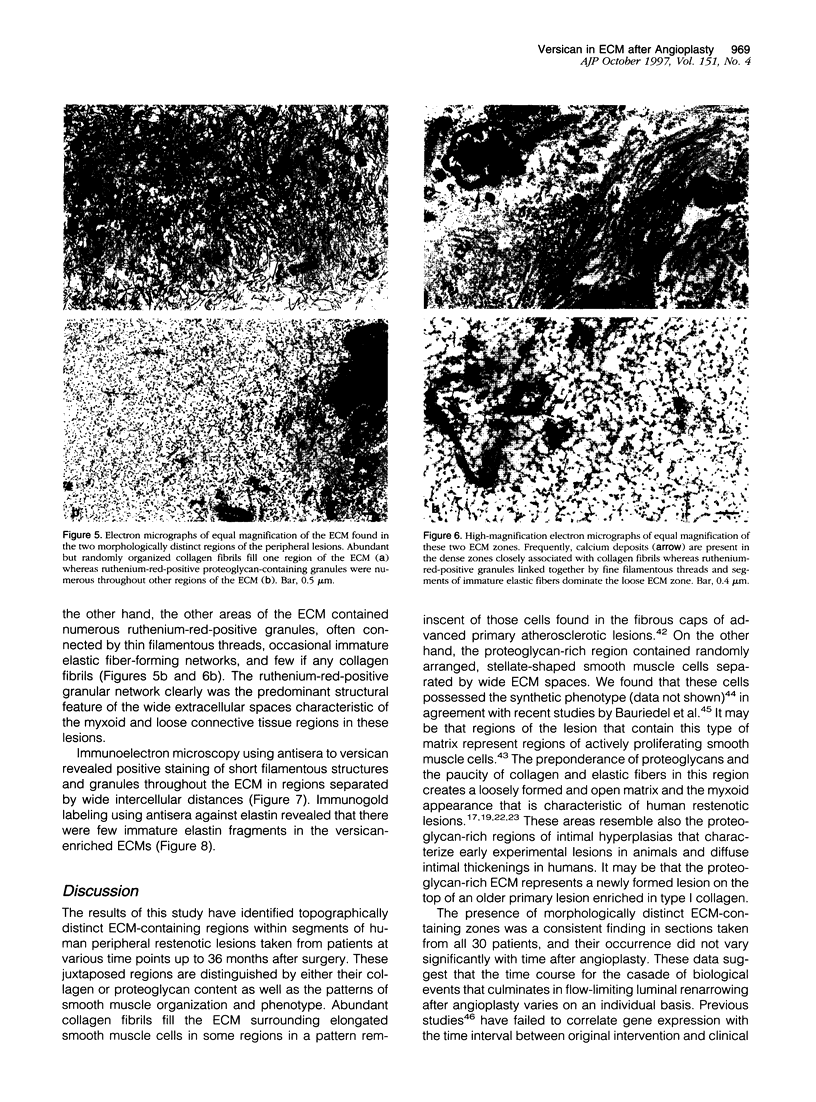
Although a large percentage of the volume of human restenotic arterial lesions is occupied by extracellular matrix (ECM), the composition and organization of this ECM are not well characterized. In this study, restenotic segments taken from 30 human peripheral arteries by directional atherectomy at times ranging from 13 days to 36 months after angioplasty were analyzed for specific patterns of ECM composition and organization by light and electron microscopic histochemistry and immunohistochemistry. Histochemical analysis revealed the presence of distinct zones, enriched either in proteoglycans or fibrillar collagen. Most sections contained these regions juxtaposed to each other. The frequency of these two distinct ECMs did not change as a function of time after angioplasty. The collagen-rich zone usually contained elongated smooth muscle cells spaced close together except in regions resembling fibrous plaques. The proteoglycan-rich ECM contained both elongated and stellate-shaped smooth muscle cells randomly arranged and separated by wide distances. This region resembled the loose-connective-tissue-containing myxoid region typical of restenotic lesions. Immunohistochemical analysis of these regions revealed that the proteoglycan-containing zone stained intensely for versican, a large interstitial chondroitin sulfate proteoglycan, whereas the collagen-containing areas were mostly negative for versican but positive for type I collagen. The versican-positive regions also immunostained for biglycan, a small leucine-rich dermatan sulfate proteoglycan, and sparsely for elastin. However, both of these ECM molecules were present in the versican-negative type I collagen-positive regions of the lesions. These results suggest that the development of restenotic lesions involves localized deposits of specific ECM molecules that may play a role in the asymmetric renarrowing of this tissue after angioplasty.
Full text
PDF
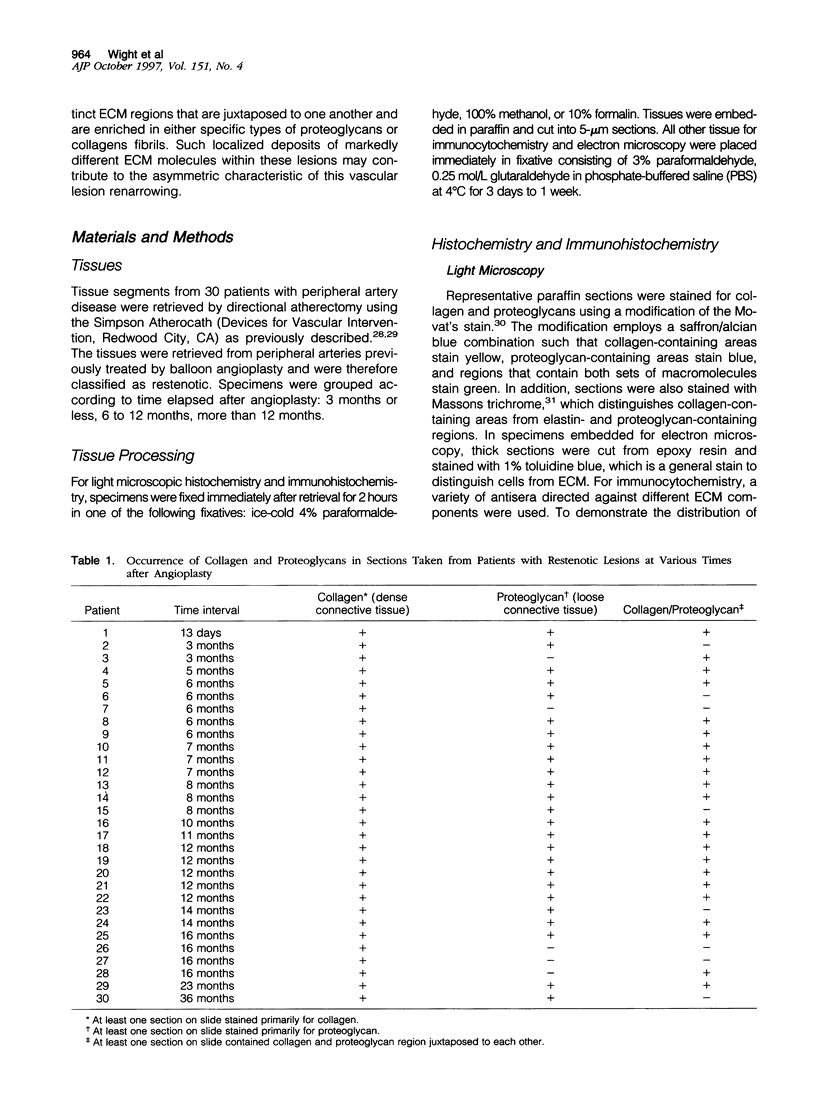

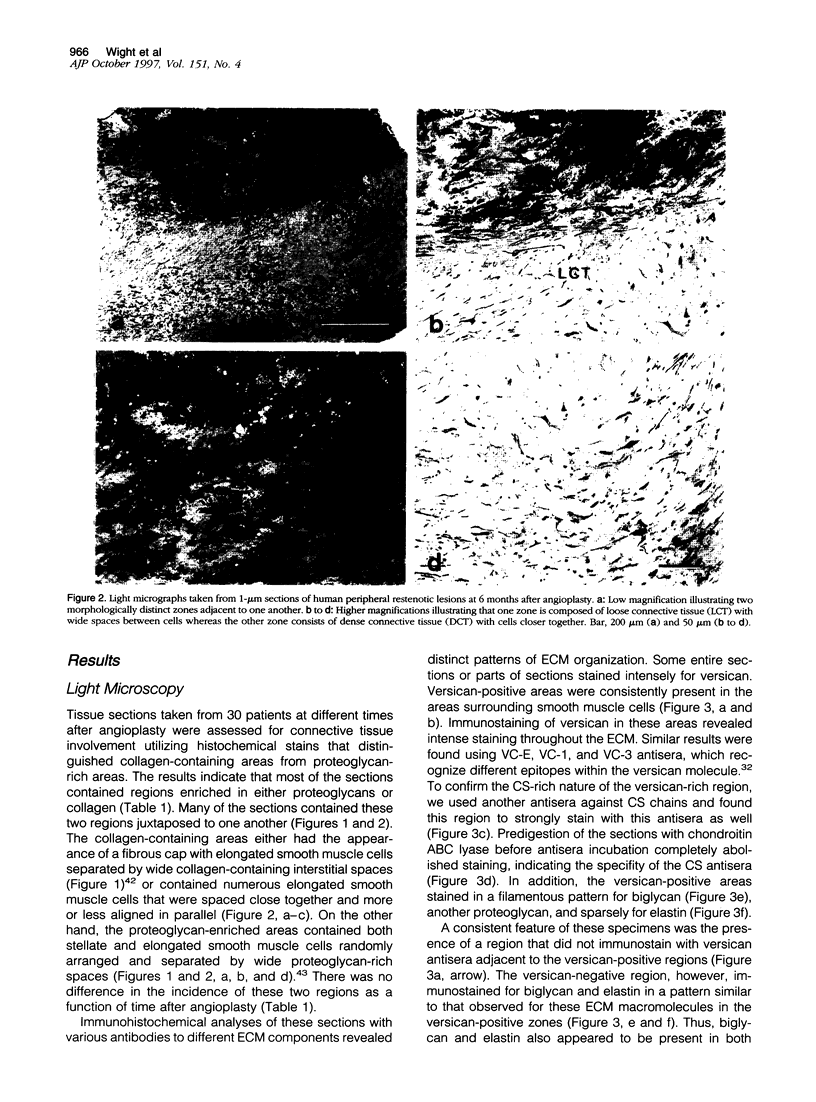
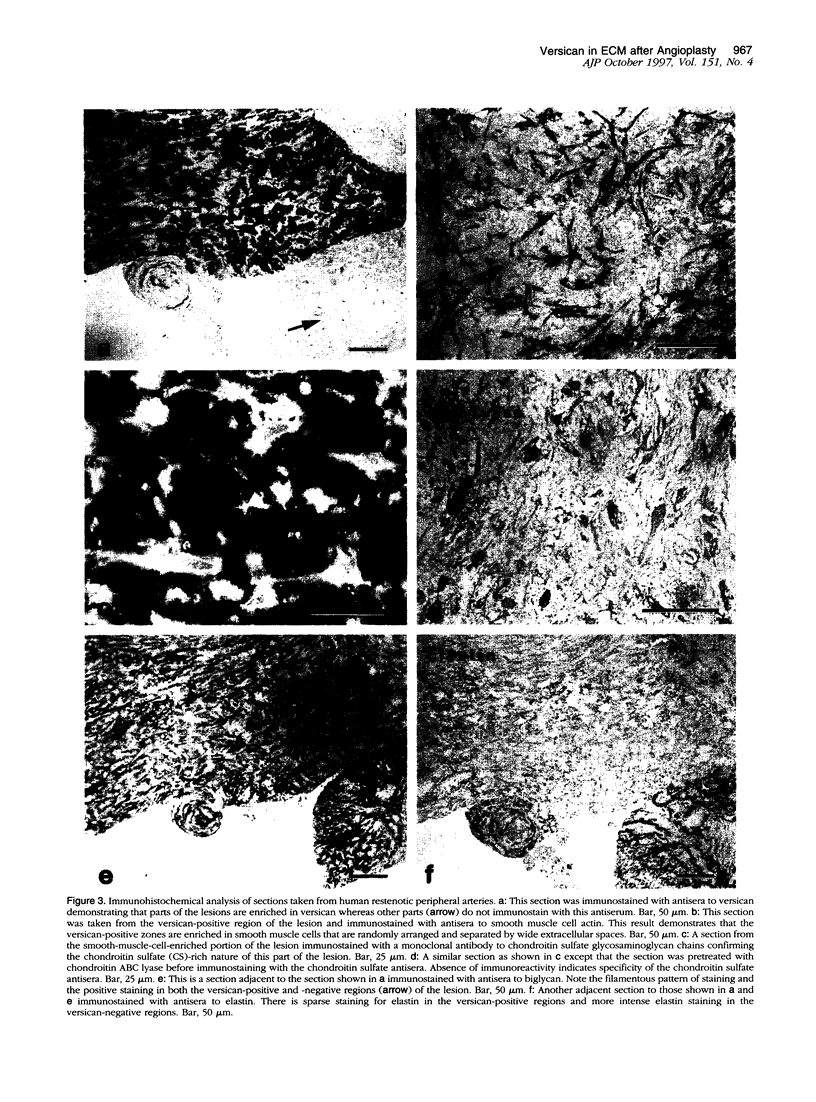




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauriedel G., Kandolf R., Schluckebier S., Welsch U. Ultrastructural characteristics of human atherectomy tissue from coronary and lower extremity arterial stenoses. Am J Cardiol. 1996 Mar 1;77(7):468–474. doi: 10.1016/s0002-9149(97)89339-3. [DOI] [PubMed] [Google Scholar]
- Campbell G. R., Campbell J. H. Smooth muscle phenotypic changes in arterial wall homeostasis: implications for the pathogenesis of atherosclerosis. Exp Mol Pathol. 1985 Apr;42(2):139–162. doi: 10.1016/0014-4800(85)90023-1. [DOI] [PubMed] [Google Scholar]
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992 Sep;86(3):723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- Chen J. K., Hoshi H., McKeehan W. L. Stimulation of human arterial smooth muscle cell chondroitin sulfate proteoglycan synthesis by transforming growth factor-beta. In Vitro Cell Dev Biol. 1991 Jan;27(1):6–12. doi: 10.1007/BF02630888. [DOI] [PubMed] [Google Scholar]
- Clausell N., de Lima V. C., Molossi S., Liu P., Turley E., Gotlieb A. I., Adelman A. G., Rabinovitch M. Expression of tumour necrosis factor alpha and accumulation of fibronectin in coronary artery restenotic lesions retrieved by atherectomy. Br Heart J. 1995 Jun;73(6):534–539. doi: 10.1136/hrt.73.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartsch P. C., Bauriedel G., Schinko I., Weiss H. D., Höfling B., Betz E. Cell constitution and characteristics of human atherosclerotic plaques selectively removed by percutaneous atherectomy. Atherosclerosis. 1989 Dec;80(2):149–157. doi: 10.1016/0021-9150(89)90023-3. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Epstein S. E., Speir E., Unger E. F., Guzman R. J., Finkel T. The basis of molecular strategies for treating coronary restenosis after angioplasty. J Am Coll Cardiol. 1994 May;23(6):1278–1288. doi: 10.1016/0735-1097(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Farb A., Virmani R., Atkinson J. B., Kolodgie F. D. Plaque morphology and pathologic changes in arteries from patients dying after coronary balloon angioplasty. J Am Coll Cardiol. 1990 Nov;16(6):1421–1429. doi: 10.1016/0735-1097(90)90386-4. [DOI] [PubMed] [Google Scholar]
- Fischell T. A. Coronary artery spasm after percutaneous transluminal coronary angioplasty: pathophysiology and clinical consequences. Cathet Cardiovasc Diagn. 1990 Jan;19(1):1–3. doi: 10.1002/ccd.1810190102. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Forrester J. S., Fishbein M., Helfant R., Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991 Mar 1;17(3):758–769. doi: 10.1016/s0735-1097(10)80196-2. [DOI] [PubMed] [Google Scholar]
- Gravanis M. B., Roubin G. S. Histopathologic phenomena at the site of percutaneous transluminal coronary angioplasty: the problem of restenosis. Hum Pathol. 1989 May;20(5):477–485. doi: 10.1016/0046-8177(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Halpert I., Sires U. I., Roby J. D., Potter-Perigo S., Wight T. N., Shapiro S. D., Welgus H. G., Wickline S. A., Parks W. C. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A. Role of platelets and thrombosis in mechanisms of acute occlusion and restenosis after angioplasty. Am J Cardiol. 1987 Jul 31;60(3):20B–28B. doi: 10.1016/0002-9149(87)90479-6. [DOI] [PubMed] [Google Scholar]
- Haude M., Erbel R., Issa H., Meyer J. Quantitative analysis of elastic recoil after balloon angioplasty and after intracoronary implantation of balloon-expandable Palmaz-Schatz stents. J Am Coll Cardiol. 1993 Jan;21(1):26–34. doi: 10.1016/0735-1097(93)90713-b. [DOI] [PubMed] [Google Scholar]
- Ip J. H., Fuster V., Badimon L., Badimon J., Taubman M. B., Chesebro J. H. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990 Jun;15(7):1667–1687. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- Isner J. M. Vascular remodeling. Honey, I think I shrunk the artery. Circulation. 1994 Jun;89(6):2937–2941. doi: 10.1161/01.cir.89.6.2937. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Hinohara T., Selmon M. R., Braden L. J., Simpson J. B. Primary peripheral arterial stenoses and restenoses excised by transluminal atherectomy: a histopathologic study. J Am Coll Cardiol. 1990 Feb;15(2):419–425. doi: 10.1016/s0735-1097(10)80071-3. [DOI] [PubMed] [Google Scholar]
- Koob T. J. Effects of chondroitinase-ABC on proteoglycans and swelling properties of fibrocartilage in bovine flexor tendon. J Orthop Res. 1989;7(2):219–227. doi: 10.1002/jor.1100070209. [DOI] [PubMed] [Google Scholar]
- Kuntz R. E., Baim D. S. Defining coronary restenosis. Newer clinical and angiographic paradigms. Circulation. 1993 Sep;88(3):1310–1323. doi: 10.1161/01.cir.88.3.1310. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Yeo T. K., Mar H., Lara S., Hellström I., Hellström K. E., Wight T. N. Arterial chondroitin sulfate proteoglycan: localization with a monoclonal antibody. J Histochem Cytochem. 1988 Oct;36(10):1211–1221. doi: 10.1177/36.10.3047228. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Fraser J. R. Hyaluronan. FASEB J. 1992 Apr;6(7):2397–2404. [PubMed] [Google Scholar]
- LeBaron R. G., Zimmermann D. R., Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992 May 15;267(14):10003–10010. [PubMed] [Google Scholar]
- Lin H., Wilson J. E., Roberts C. R., Horley K. J., Winters G. L., Costanzo M. R., McManus B. M. Biglycan, decorin, and versican protein expression patterns in coronary arteriopathy of human cardiac allograft: distinctness as compared to native atherosclerosis. J Heart Lung Transplant. 1996 Dec;15(12):1233–1247. [PubMed] [Google Scholar]
- Lipshitz H., Etheredge R., 3rd, Glimcher M. J. Changes in the hexosamine content and swelling ratio of articular cartilage as functions of depth from the surface. J Bone Joint Surg Am. 1976 Dec;58(8):1149–1153. [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar H., Wight T. N. Colloidal gold immunostaining on deplasticized ultra-thin sections. J Histochem Cytochem. 1988 Nov;36(11):1387–1395. doi: 10.1177/36.11.2844888. [DOI] [PubMed] [Google Scholar]
- Maroudas A. I. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976 Apr 29;260(5554):808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- Matsuura R., Isaka N., Imanaka-Yoshida K., Yoshida T., Sakakura T., Nakano T. Deposition of PG-M/versican is a major cause of human coronary restenosis after percutaneous transluminal coronary angioplasty. J Pathol. 1996 Nov;180(3):311–316. doi: 10.1002/(SICI)1096-9896(199611)180:3<311::AID-PATH657>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Muller D. W., Ellis S. G., Topol E. J. Experimental models of coronary artery restenosis. J Am Coll Cardiol. 1992 Feb;19(2):418–432. doi: 10.1016/0735-1097(92)90500-m. [DOI] [PubMed] [Google Scholar]
- Nikkari S. T., Clowes A. W. Restenosis after vascular reconstruction. Ann Med. 1994 Apr;26(2):95–100. doi: 10.3109/07853899409147335. [DOI] [PubMed] [Google Scholar]
- Nikol S., Isner J. M., Pickering J. G., Kearney M., Leclerc G., Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J Clin Invest. 1992 Oct;90(4):1582–1592. doi: 10.1172/JCI116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuyoshi M., Kimura T., Ohishi H., Horiuchi H., Nosaka H., Hamasaki N., Yokoi H., Kim K. Restenosis after percutaneous transluminal coronary angioplasty: pathologic observations in 20 patients. J Am Coll Cardiol. 1991 Feb;17(2):433–439. doi: 10.1016/s0735-1097(10)80111-1. [DOI] [PubMed] [Google Scholar]
- Oksala O., Salo T., Tammi R., Häkkinen L., Jalkanen M., Inki P., Larjava H. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem. 1995 Feb;43(2):125–135. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- Pickering J. G., Weir L., Jekanowski J., Kearney M. A., Isner J. M. Proliferative activity in peripheral and coronary atherosclerotic plaque among patients undergoing percutaneous revascularization. J Clin Invest. 1993 Apr;91(4):1469–1480. doi: 10.1172/JCI116352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Vande Berg J., Rudolph R., Tarpley J., Mustoe T. A. Platelet-derived growth factor-BB and transforming growth factor beta 1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol. 1991 Mar;138(3):629–646. [PMC free article] [PubMed] [Google Scholar]
- Post M. J., Borst C., Kuntz R. E. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. A study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation. 1994 Jun;89(6):2816–2821. doi: 10.1161/01.cir.89.6.2816. [DOI] [PubMed] [Google Scholar]
- Riessen R., Isner J. M., Blessing E., Loushin C., Nikol S., Wight T. N. Regional differences in the distribution of the proteoglycans biglycan and decorin in the extracellular matrix of atherosclerotic and restenotic human coronary arteries. Am J Pathol. 1994 May;144(5):962–974. [PMC free article] [PubMed] [Google Scholar]
- Riessen R., Wight T. N., Pastore C., Henley C., Isner J. M. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation. 1996 Mar 15;93(6):1141–1147. doi: 10.1161/01.cir.93.6.1141. [DOI] [PubMed] [Google Scholar]
- Ross R., Wight T. N., Strandness E., Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984 Jan;114(1):79–93. [PMC free article] [PubMed] [Google Scholar]
- Safian R. D., Gelbfish J. S., Erny R. E., Schnitt S. J., Schmidt D. A., Baim D. S. Coronary atherectomy. Clinical, angiographic, and histological findings and observations regarding potential mechanisms. Circulation. 1990 Jul;82(1):69–79. doi: 10.1161/01.cir.82.1.69. [DOI] [PubMed] [Google Scholar]
- Salustri A., Yanagishita M., Underhill C. B., Laurent T. C., Hascall V. C. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev Biol. 1992 Jun;151(2):541–551. doi: 10.1016/0012-1606(92)90192-j. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Holmes D. R., Jr, Topol E. J. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J Am Coll Cardiol. 1992 Nov 1;20(5):1284–1293. doi: 10.1016/0735-1097(92)90389-5. [DOI] [PubMed] [Google Scholar]
- Schönherr E., Järveläinen H. T., Kinsella M. G., Sandell L. J., Wight T. N. Platelet-derived growth factor and transforming growth factor-beta 1 differentially affect the synthesis of biglycan and decorin by monkey arterial smooth muscle cells. Arterioscler Thromb. 1993 Jul;13(7):1026–1036. doi: 10.1161/01.atv.13.7.1026. [DOI] [PubMed] [Google Scholar]
- Schönherr E., Järveläinen H. T., Sandell L. J., Wight T. N. Effects of platelet-derived growth factor and transforming growth factor-beta 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991 Sep 15;266(26):17640–17647. [PubMed] [Google Scholar]
- Simpson J. B., Selmon M. R., Robertson G. C., Cipriano P. R., Hayden W. G., Johnson D. E., Fogarty T. J. Transluminal atherectomy for occlusive peripheral vascular disease. Am J Cardiol. 1988 May 9;61(14):96G–101G. doi: 10.1016/s0002-9149(88)80040-7. [DOI] [PubMed] [Google Scholar]
- Topol E. J. The restenosis "antitheory". Mayo Clin Proc. 1993 Jan;68(1):88–90. doi: 10.1016/s0025-6196(12)60026-3. [DOI] [PubMed] [Google Scholar]
- Tyagi S. C., Meyer L., Schmaltz R. A., Reddy H. K., Voelker D. J. Proteinases and restenosis in the human coronary artery: extracellular matrix production exceeds the expression of proteolytic activity. Atherosclerosis. 1995 Jul;116(1):43–57. doi: 10.1016/0021-9150(95)05520-7. [DOI] [PubMed] [Google Scholar]
- Ueda M., Becker A. E., Fujimoto T. Pathological changes induced by repeated percutaneous transluminal coronary angioplasty. Br Heart J. 1987 Dec;58(6):635–643. doi: 10.1136/hrt.58.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight T. N., Kinsella M. G., Qwarnström E. E. The role of proteoglycans in cell adhesion, migration and proliferation. Curr Opin Cell Biol. 1992 Oct;4(5):793–801. doi: 10.1016/0955-0674(92)90102-i. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Ross R. Proteoglycans in primate arteries. I. Ultrastructural localization and distribution in the intima. J Cell Biol. 1975 Dec;67(3):660–674. doi: 10.1083/jcb.67.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo T. K., Brown L., Dvorak H. F. Alterations in proteoglycan synthesis common to healing wounds and tumors. Am J Pathol. 1991 Jun;138(6):1437–1450. [PMC free article] [PubMed] [Google Scholar]
- Yeo T. K., Macfarlane S., Wight T. N. Characterization of a chondroitin sulfate proteoglycan synthesized by monkey arterial smooth muscle cells in vitro. Connect Tissue Res. 1992;27(4):265–277. doi: 10.3109/03008209209007001. [DOI] [PubMed] [Google Scholar]