Abstract
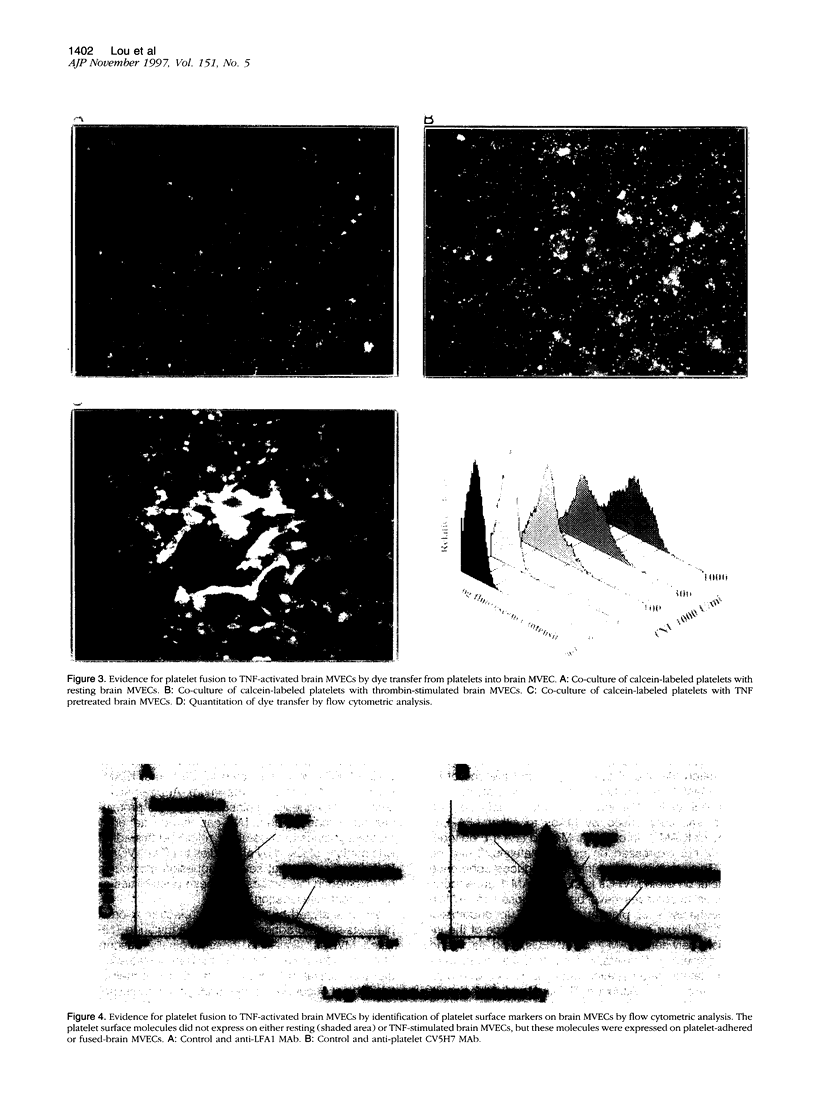
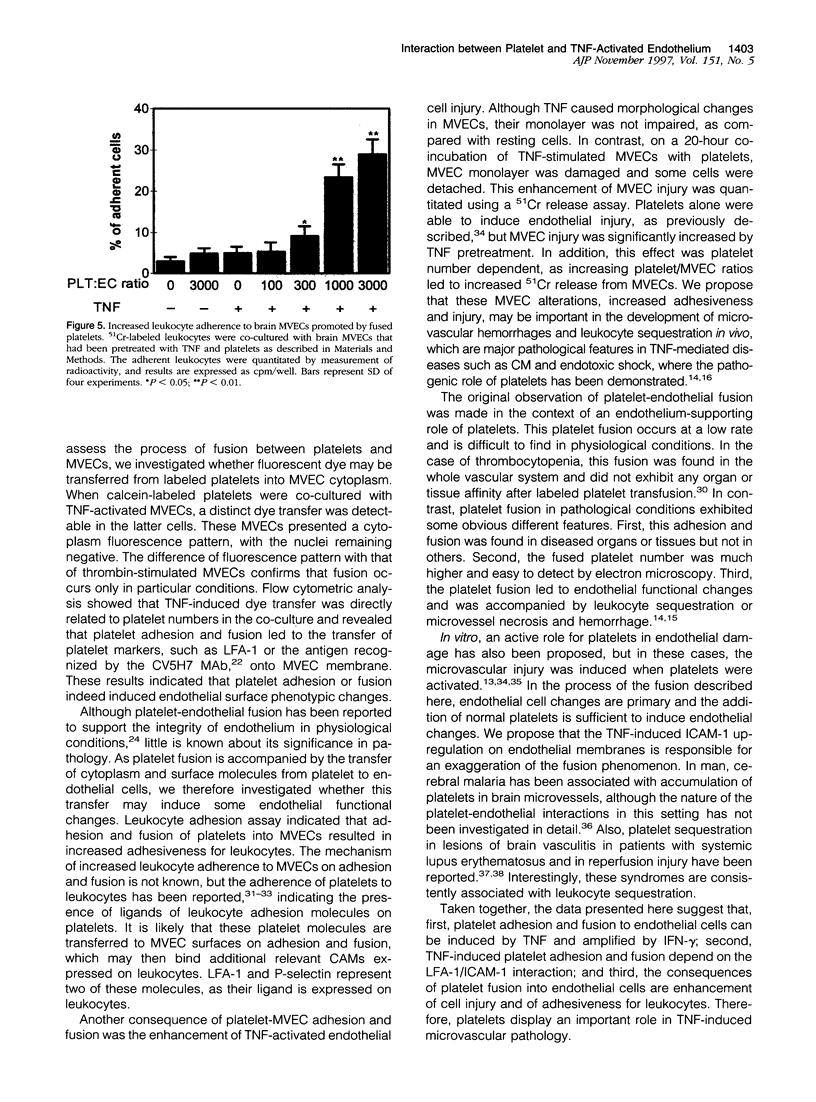
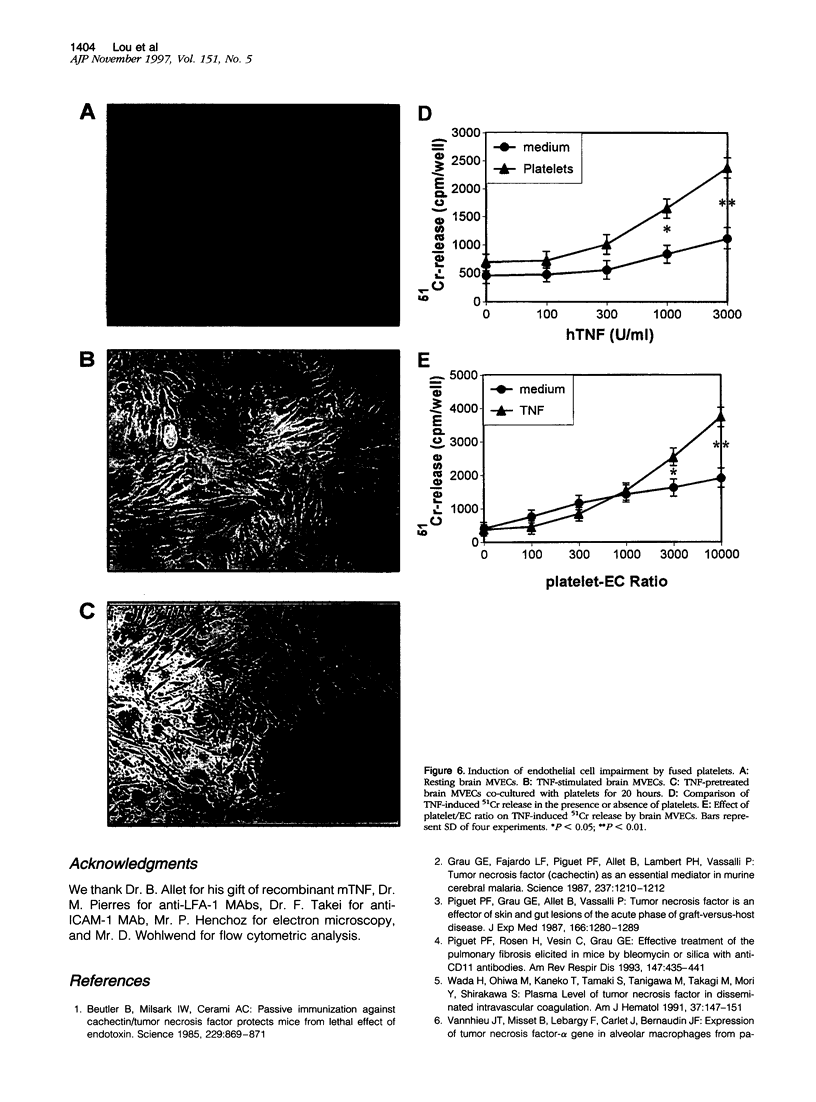
Tumor necrosis factor-alpha (TNF) is known to be an important mediator in the pathogenesis of several inflammatory diseases. Vascular endothelial cells represent a major target of TNF effects. Platelet sequestration has been found in brain microvessels during experimental cerebral malaria and lung in experimental pulmonary fibrosis, implying that it may participate in TNF-dependent microvascular pathology. In this study, we investigated the mechanisms of platelet-endothelial interaction, using co-cultures between platelets and TNF-activated mouse brain microvascular endothelial cells (MVECs). Adhesion and fusion of platelets to MVECs was evidenced by electron microscopy, dye transfer, and flow cytometry. It was induced by TNF and interferon-gamma and depended on LFA-1 expressed on the platelet surface and ICAM-1 expressed on MVECs. The adhesion and fusion also led to the transfer of platelet markers on the MVEC surface, rendering these more adherent for leukocytes, and to an enhanced MVEC sensitivity to TNF-induced injury. These results suggest that platelets can participate in TNF-induced microvascular pathology.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avraham H., Cowley S., Chi S. Y., Jiang S., Groopman J. E. Characterization of adhesive interactions between human endothelial cells and megakaryocytes. J Clin Invest. 1993 Jun;91(6):2378–2384. doi: 10.1172/JCI116470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni P. N., Carney D. H., Nicolson G. L. Organ-derived microvessel endothelial cells exhibit differential responsiveness to thrombin and other growth factors. Microvasc Res. 1992 Jan;43(1):20–45. doi: 10.1016/0026-2862(92)90004-9. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Cotran R. S. American Association of Pathologists president's address. New roles for the endothelium in inflammation and immunity. Am J Pathol. 1987 Dec;129(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- Cywes R., Packham M. A., Tietze L., Sanabria J. R., Harvey P. R., Phillips M. J., Strasberg S. M. Role of platelets in hepatic allograft preservation injury in the rat. Hepatology. 1993 Sep;18(3):635–647. [PubMed] [Google Scholar]
- Ellison D., Gatter K., Heryet A., Esiri M. Intramural platelet deposition in cerebral vasculopathy of systemic lupus erythematosus. J Clin Pathol. 1993 Jan;46(1):37–40. doi: 10.1136/jcp.46.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Hajjar D. P. von Willebrand factor mediates platelet adhesion to virally infected endothelial cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5153–5156. doi: 10.1073/pnas.90.11.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti S., Faggioni R., Echtenacher B., Ghezzi P. Role of tumour necrosis factor and reactive oxygen intermediates in lipopolysaccharide-induced pulmonary oedema and lethality. Clin Exp Immunol. 1993 Mar;91(3):456–461. doi: 10.1111/j.1365-2249.1993.tb05924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Aster R. H., Cotran R. S., Corkery J., Jandl J. H., Folkman J. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature. 1969 Apr 5;222(5188):33–36. doi: 10.1038/222033a0. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Lou J. TNF in vascular pathology: the importance of platelet-endothelium interactions. Res Immunol. 1993 Jun;144(5):355–363. doi: 10.1016/s0923-2494(93)80080-i. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Gretener D., Vesin C., Lambert P. H. Immunopathology of thrombocytopenia in experimental malaria. Immunology. 1988 Dec;65(4):501–506. [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Pointaire P., Piguet P. F., Vesin C., Rosen H., Stamenkovic I., Takei F., Vassalli P. Late administration of monoclonal antibody to leukocyte function-antigen 1 abrogates incipient murine cerebral malaria. Eur J Immunol. 1991 Sep;21(9):2265–2267. doi: 10.1002/eji.1830210939. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Tacchini-Cottier F., Vesin C., Milon G., Lou J. N., Piguet P. F., Juillard P. TNF-induced microvascular pathology: active role for platelets and importance of the LFA-1/ICAM-1 interaction. Eur Cytokine Netw. 1993 Nov-Dec;4(6):415–419. [PubMed] [Google Scholar]
- Herbaczyńska-Cedro K., Lembowicz K., Pytel B. NG-monomethyl-L-arginine increases platelet deposition on damaged endothelium in vivo. A scanning electron microscopic study. Thromb Res. 1991 Oct 1;64(1):1–9. doi: 10.1016/0049-3848(91)90200-g. [DOI] [PubMed] [Google Scholar]
- Hirafuji M., Shinoda H. PAF-mediated platelet adhesion to endothelial cells induced by FMLP-stimulated leukocytes. J Lipid Mediat. 1991 Nov;4(3):347–351. [PubMed] [Google Scholar]
- JOHNSON S. A., BALBOA R. S., DESSEL B. H., MONTO R. W., SIEGESMUND K. A., GREENWALT T. J. THE MECHANISM OF THE ENDOTHELIAL SUPPORTING FUNCTION OF INTACT PLATELETS. Exp Mol Pathol. 1964 Apr;34:115–127. doi: 10.1016/0014-4800(64)90045-0. [DOI] [PubMed] [Google Scholar]
- Jørgensen L., Grøthe A. G., Larsen T., Kinlough-Rathbone R. L., Mustard J. F. Injury to cultured endothelial cells by thrombin-stimulated platelets. Lab Invest. 1986 Apr;54(4):408–415. [PubMed] [Google Scholar]
- Kahan A., Amor B., Menkes C. J., Strauch G. Recombinant interferon-gamma in the treatment of systemic sclerosis. Am J Med. 1989 Sep;87(3):273–277. doi: 10.1016/s0002-9343(89)80150-0. [DOI] [PubMed] [Google Scholar]
- Kishi Y., Numano F. In vitro study of vascular endothelial injury by activated platelets and its prevention. Atherosclerosis. 1989 Apr;76(2-3):95–101. doi: 10.1016/0021-9150(89)90092-0. [DOI] [PubMed] [Google Scholar]
- McCaffery P. J., Berridge M. V. Expression of the leukocyte functional molecule (LFA-1) on mouse platelets. Blood. 1986 Jun;67(6):1757–1764. [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Allet B., Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987 Nov 1;166(5):1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Rosen H., Vesin C., Grau G. E. Effective treatment of the pulmonary fibrosis elicited in mice by bleomycin or silica with anti-CD-11 antibodies. Am Rev Respir Dis. 1993 Feb;147(2):435–441. doi: 10.1164/ajrccm/147.2.435. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Vesin C. Pulmonary platelet trapping induced by bleomycin: correlation with fibrosis and involvement of the beta 2 integrins. Int J Exp Pathol. 1994 Oct;75(5):321–328. [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Vesin C., Ryser J. E., Senaldi G., Grau G. E., Tacchini-Cottier F. An effector role for platelets in systemic and local lipopolysaccharide-induced toxicity in mice, mediated by a CD11a- and CD54-dependent interaction with endothelium. Infect Immun. 1993 Oct;61(10):4182–4187. doi: 10.1128/iai.61.10.4182-4187.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Pongponratn E., Riganti M., Harinasuta T., Bunnag D. Electron microscopy of the human brain in cerebral malaria. Southeast Asian J Trop Med Public Health. 1985 Jun;16(2):219–227. [PubMed] [Google Scholar]
- Roberts D. J., Davies J. M., Evans C. C., Bell M., Mostafa S. M., Lamche H. Tumour necrosis factor and adult respiratory distress syndrome. Lancet. 1989 Oct 28;2(8670):1043–1044. doi: 10.1016/s0140-6736(89)91058-1. [DOI] [PubMed] [Google Scholar]
- Rupnick M. A., Carey A., Williams S. K. Phenotypic diversity in cultured cerebral microvascular endothelial cells. In Vitro Cell Dev Biol. 1988 May;24(5):435–444. doi: 10.1007/BF02628495. [DOI] [PubMed] [Google Scholar]
- Schulze C., Firth J. A. Immunohistochemical localization of adherens junction components in blood-brain barrier microvessels of the rat. J Cell Sci. 1993 Mar;104(Pt 3):773–782. doi: 10.1242/jcs.104.3.773. [DOI] [PubMed] [Google Scholar]
- Spangenberg P., Redlich H., Bergmann I., Lösche W., Götzrath M., Kehrel B. The platelet glycoprotein IIb/IIIa complex is involved in the adhesion of activated platelets to leukocytes. Thromb Haemost. 1993 Sep 1;70(3):514–521. [PubMed] [Google Scholar]
- Todoroki N., Watanabe Y., Akaike T., Katagiri Y., Tanoue K., Yamazaki H., Tsuji T., Toyoshima S., Osawa T. Enhancement by IL-1 beta and IFN-gamma of platelet activation: adhesion to leukocytes via GMP-140/PADGEM protein (CD62). Biochem Biophys Res Commun. 1991 Sep 16;179(2):756–761. doi: 10.1016/0006-291x(91)91881-c. [DOI] [PubMed] [Google Scholar]
- Wada H., Ohiwa M., Kaneko T., Tamaki S., Tanigawa M., Takagi M., Mori Y., Shirakawa S. Plasma level of tumor necrosis factor in disseminated intravascular coagulation. Am J Hematol. 1991 Jul;37(3):147–151. doi: 10.1002/ajh.2830370302. [DOI] [PubMed] [Google Scholar]
- Wojcik J. D., Van Horn D. L., Webber A. J., Johnson S. A. Mechanism whereby platelets support the endothelium. Transfusion. 1969 Nov-Dec;9(6):324–335. doi: 10.1111/j.1537-2995.1969.tb04945.x. [DOI] [PubMed] [Google Scholar]
- Woodley N., Barclay J. K. Cultured endothelial cells from distinct vascular areas show differential responses to agonists. Can J Physiol Pharmacol. 1994 Sep;72(9):1007–1012. doi: 10.1139/y94-140. [DOI] [PubMed] [Google Scholar]
- Yamazaki H., Fujimoto T., Suzuki H., Akamatsu N., Katagiri Y., Yamaguchi A., Tanoue K. Interaction of platelets and blood vessels--vascular injuries induced by platelet activation in vivo. Jpn Circ J. 1992 Feb;56(2):178–186. doi: 10.1253/jcj.56.178. [DOI] [PubMed] [Google Scholar]
- de Bruijne-Admiraal L. G., Modderman P. W., Von dem Borne A. E., Sonnenberg A. P-selectin mediates Ca(2+)-dependent adhesion of activated platelets to many different types of leukocytes: detection by flow cytometry. Blood. 1992 Jul 1;80(1):134–142. [PubMed] [Google Scholar]