Abstract
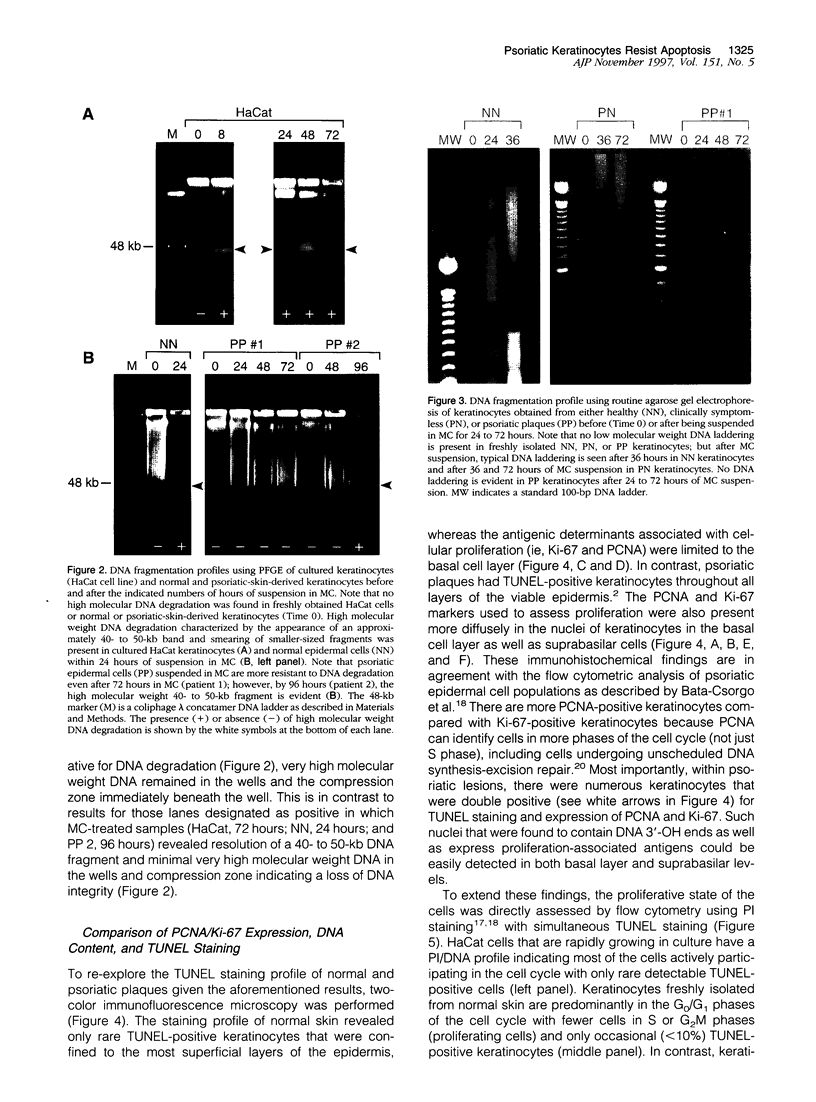
Previously we observed that hyperplastic epidermal keratinocytes characteristic of psoriasis had abundant amounts of the cell survival protein Bcl-xL; however, whether this overexpression correlated with enhanced survival was unclear because the majority of epidermal cells possess nuclei that are positively labeled by an assay typically regarded as indicative of cells undergoing apoptosis (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) staining). To clarify this apparent discrepancy, we explored the propensity of keratinocytes derived from psoriatic plaques to undergo apoptosis and also determined the reliability of TUNEL staining as an indicator of apoptosis in keratinocytes in vitro and in vivo. First, a keratinocyte cell line, HaCat, was examined before and after being suspended in semisolid medium (methylcellulose) using flow cytometry to detect TUNEL-positive cells, and the percentage of positive cells was correlated to the presence or absence of double-stranded DNA fragmentation using pulsed field gel electrophoresis. After 18 hours in methylcellulose suspension, apoptosis was detected in HaCat cells when at least 5% of the cell population was undergoing programmed cell death. Second, we examined 23 clinical specimens of skin (13 from psoriatic patients and 10 from healthy control subjects) and observed that no double-stranded DNA fragmentation was present in any of the freshly isolated keratinocytes from either normal or psoriatic patients. Keratinocytes from 9 of 12 normal skin samples underwent double-stranded DNA fragmentation after being in methylcellulose for 18 to 24 hours, which contrasts with keratinocytes from lesions of psoriasis where only 1 of 13 of the skin samples had these changes. Third, two-color immunofluorescence staining of psoriatic plaques revealed that numerous TUNEL-positive keratinocytes were also positive for proliferating cell nuclear antigen and Ki-67 antigens and that by flow cytometry TUNEL-positive keratinocytes obtained from psoriatic plaques possessed a DNA content profile indicative of proliferating and not dying cells. These results demonstrate that keratinocytes within psoriatic plaques do not have double-stranded DNA breaks, that they have a prolonged capacity to resist induction of apoptosis compared with normal-skin-derived keratinocytes or cultured HaCat cells, and that caution is necessary for proper interpretation related to detection of 3'-OH DNA ends (ie, TUNEL positivity) in skin, as it can be associated with DNA synthesis as well as cell death.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bata-Csorgo Z., Hammerberg C., Voorhees J. J., Cooper K. D. Flow cytometric identification of proliferative subpopulations within normal human epidermis and the localization of the primary hyperproliferative population in psoriasis. J Exp Med. 1993 Oct 1;178(4):1271–1281. doi: 10.1084/jem.178.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstresser P. R., Taylor J. R. Epidermal 'turnover time'--a new examination. Br J Dermatol. 1977 May;96(5):503–509. doi: 10.1111/j.1365-2133.1977.tb07152.x. [DOI] [PubMed] [Google Scholar]
- Bianchi L., Farrace M. G., Nini G., Piacentini M. Abnormal Bcl-2 and "tissue" transglutaminase expression in psoriatic skin. J Invest Dermatol. 1994 Dec;103(6):829–833. doi: 10.1111/1523-1747.ep12413590. [DOI] [PubMed] [Google Scholar]
- Bohr V., Mansbridge J., Hanawalt P. Comparative effects of growth inhibitors on DNA replication, DNA repair, and protein synthesis in human epidermal keratinocytes. Cancer Res. 1986 Jun;46(6):2929–2935. [PubMed] [Google Scholar]
- Celis J. E., Madsen P. Increased nuclear cyclin/PCNA antigen staining of non S-phase transformed human amnion cells engaged in nucleotide excision DNA repair. FEBS Lett. 1986 Dec 15;209(2):277–283. doi: 10.1016/0014-5793(86)81127-9. [DOI] [PubMed] [Google Scholar]
- Dole M. G., Jasty R., Cooper M. J., Thompson C. B., Nuñez G., Castle V. P. Bcl-xL is expressed in neuroblastoma cells and modulates chemotherapy-induced apoptosis. Cancer Res. 1995 Jun 15;55(12):2576–2582. [PubMed] [Google Scholar]
- Elder J. T., Fisher G. J., Lindquist P. B., Bennett G. L., Pittelkow M. R., Coffey R. J., Jr, Ellingsworth L., Derynck R., Voorhees J. J. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989 Feb 10;243(4892):811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca W., Bigman K., Mittelman A., Ahmed T., Gong J., Melamed M. R., Darzynkiewicz Z. Induction of DNA strand breaks associated with apoptosis during treatment of leukemias. Leukemia. 1993 May;7(5):659–670. [PubMed] [Google Scholar]
- Gottlieb A. B., Chang C. K., Posnett D. N., Fanelli B., Tam J. P. Detection of transforming growth factor alpha in normal, malignant, and hyperproliferative human keratinocytes. J Exp Med. 1988 Feb 1;167(2):670–675. doi: 10.1084/jem.167.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake A. R., Polakowska R. R. Cell death by apoptosis in epidermal biology. J Invest Dermatol. 1993 Aug;101(2):107–112. doi: 10.1111/1523-1747.ep12363594. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Hengartner M. O. Programmed cell death. A rich harvest. Curr Biol. 1994 Oct 1;4(10):950–952. doi: 10.1016/s0960-9822(00)00216-5. [DOI] [PubMed] [Google Scholar]
- Hines M. D., Allen-Hoffmann B. L. Keratinocyte growth factor inhibits cross-linked envelope formation and nucleosomal fragmentation in cultured human keratinocytes. J Biol Chem. 1996 Mar 15;271(11):6245–6251. doi: 10.1074/jbc.271.11.6245. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Wolfe J. T., Nabeya R. T., Vallat V. P., Gilleaudeau P., Heftler N. S., Austin L. M., Gottlieb A. B. Successful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology and by selective depletion of intraepidermal T cells. J Exp Med. 1995 Dec 1;182(6):2057–2068. doi: 10.1084/jem.182.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E. M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986 Sep;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Leigh I. M., Navsaria H., Purkis P. E., McKay I. A., Bowden P. E., Riddle P. N. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol. 1995 Oct;133(4):501–511. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- Liang X. H., Mungal S., Ayscue A., Meissner J. D., Wodnicki P., Hockenbery D., Lockett S., Herman B. Bcl-2 protooncogene expression in cervical carcinoma cell lines containing inactive p53. J Cell Biochem. 1995 Mar;57(3):509–521. doi: 10.1002/jcb.240570316. [DOI] [PubMed] [Google Scholar]
- McCall C. A., Cohen J. J. Programmed cell death in terminally differentiating keratinocytes: role of endogenous endonuclease. J Invest Dermatol. 1991 Jul;97(1):111–114. doi: 10.1111/1523-1747.ep12478519. [DOI] [PubMed] [Google Scholar]
- McCormick D., Hall P. A. The complexities of proliferating cell nuclear antigen. Histopathology. 1992 Dec;21(6):591–594. doi: 10.1111/j.1365-2559.1992.tb00454.x. [DOI] [PubMed] [Google Scholar]
- McKay I. A., Leigh I. M. Altered keratinocyte growth and differentiation in psoriasis. Clin Dermatol. 1995 Mar-Apr;13(2):105–114. doi: 10.1016/0738-081x(95)93817-8. [DOI] [PubMed] [Google Scholar]
- Mitra R. S., Wrone-Smith T., Simonian P., Foreman K. E., Nunez G., Nickoloff B. J. Apoptosis in keratinocytes is not dependent on induction of differentiation. Lab Invest. 1997 Jan;76(1):99–107. [PubMed] [Google Scholar]
- Nickoloff B. J., Fisher G. J., Mitra R. S., Voorhees J. J. Additive and synergistic antiproliferative effects of cyclosporin A and gamma interferon on cultured human keratinocytes. Am J Pathol. 1988 Apr;131(1):12–18. [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivetti G., Abbi R., Quaini F., Kajstura J., Cheng W., Nitahara J. A., Quaini E., Di Loreto C., Beltrami C. A., Krajewski S. Apoptosis in the failing human heart. N Engl J Med. 1997 Apr 17;336(16):1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- Pandey S., Wang E. Cells en route to apoptosis are characterized by the upregulation of c-fos, c-myc, c-jun, cdc2, and RB phosphorylation, resembling events of early cell-cycle traverse. J Cell Biochem. 1995 Jun;58(2):135–150. doi: 10.1002/jcb.240580203. [DOI] [PubMed] [Google Scholar]
- Polakowska R. R., Haake A. R. Apoptosis: the skin from a new perspective. Cell Death Differ. 1994 Jul;1(1):19–31. [PubMed] [Google Scholar]
- Potten C. S., Hendry J. H. Letter: Clonogenic cells and stem cells in epidermis. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Nov;24(5):537–540. doi: 10.1080/09553007314551441. [DOI] [PubMed] [Google Scholar]
- Ryle C. M., Breitkreutz D., Stark H. J., Leigh I. M., Steinert P. M., Roop D., Fusenig N. E. Density-dependent modulation of synthesis of keratins 1 and 10 in the human keratinocyte line HACAT and in ras-transfected tumorigenic clones. Differentiation. 1989 Mar;40(1):42–54. doi: 10.1111/j.1432-0436.1989.tb00812.x. [DOI] [PubMed] [Google Scholar]
- Sachsenmeier K. F., Sheibani N., Schlosser S. J., Allen-Hoffmann B. L. Transforming growth factor-beta 1 inhibits nucleosomal fragmentation in human keratinocytes following loss of adhesion. J Biol Chem. 1996 Jan 5;271(1):5–8. doi: 10.1074/jbc.271.1.5. [DOI] [PubMed] [Google Scholar]
- Sadek C. M., Allen-Hoffmann B. L. Cytochrome P450IA1 is rapidly induced in normal human keratinocytes in the absence of xenobiotics. J Biol Chem. 1994 Jun 10;269(23):16067–16074. [PubMed] [Google Scholar]
- Seiberg M., Marthinuss J., Stenn K. S. Changes in expression of apoptosis-associated genes in skin mark early catagen. J Invest Dermatol. 1995 Jan;104(1):78–82. doi: 10.1111/1523-1747.ep12613555. [DOI] [PubMed] [Google Scholar]
- Smith G. H., Sharp R., Kordon E. C., Jhappan C., Merlino G. Transforming growth factor-alpha promotes mammary tumorigenesis through selective survival and growth of secretory epithelial cells. Am J Pathol. 1995 Oct;147(4):1081–1096. [PMC free article] [PubMed] [Google Scholar]
- VANSCOTT E. J., EKEL T. M. KINETICS OF HYPERPLASIA IN PSORIASIS. Arch Dermatol. 1963 Oct;88:373–381. [PubMed] [Google Scholar]
- Wrone-Smith T., Johnson T., Nelson B., Boise L. H., Thompson C. B., Núez G., Nickoloff B. J. Discordant expression of Bcl-x and Bcl-2 by keratinocytes in vitro and psoriatic keratinocytes in vivo. Am J Pathol. 1995 May;146(5):1079–1088. [PMC free article] [PubMed] [Google Scholar]






