Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease with primary manifestations in the synovial membrane. Tissue infiltrates are composed of T cells, B cells, and macrophages, but histopathological appearances vary widely and are rarely pathognomonic. Mechanisms underlying the phenotypic heterogeneity of rheumatoid synovitis are not known. To explore whether a correlation exists between the microscopic patterns of rheumatoid synovitis and in situ production of cytokines, tissue samples from 21 consecutive patients with clinically active RA were examined. Based upon the organization of the lymphocyte infiltrate, the synovial biopsies were categorized into three distinct subsets. Ten samples were characterized by diffuse lymphoid infiltrates without further microarrangement. In seven samples, lymphoid follicles with germinal center formation were detected, and in four specimens, granuloma formation was identified. In all specimens, cytokine transcription of interferon (IFN)-gamma, interleukin (IL)-4, IL-1 beta, tumor necrosis factor (TNF)-alpha, IL-10, and transforming growth factor-beta 1 was semiquantified with polymerase chain reaction and liquid phase hybridization. Each of the morphologically defined variants of synovitis displayed a unique cytokine profile. Low-level transcription of IFN-gamma, IL-4, IL-1 beta, and TNF-alpha was typical of diffuse synovitis. In follicular synovitis, IFN-gamma was the dominant cytokine, IL-4 was virtually undetectable, and IL-10 was abundant. Granulomatous synovitis demonstrated high transcription of IFN-gamma, IL-4, IL-1 beta, and TNF-alpha and could be clearly distinguished from the other phenotypes. To investigate whether differences in the synovial lesions were related to host factors, patients were compared for clinical parameters. Diffuse synovitis was seen in most of the patients with seronegative RA, the mildest form of the disease. In contrast, extra-articular spreading of RA with nodule formation was typically associated with granulomatous synovitis. In summary, RA patients display reproducible patterns in the organization and activity of synovial infiltrates. The correlation of microanatomy with tissue cytokine production suggests that several pathomechanisms can modulate the expression of the immune response in the synovial membrane.
Full text
PDF


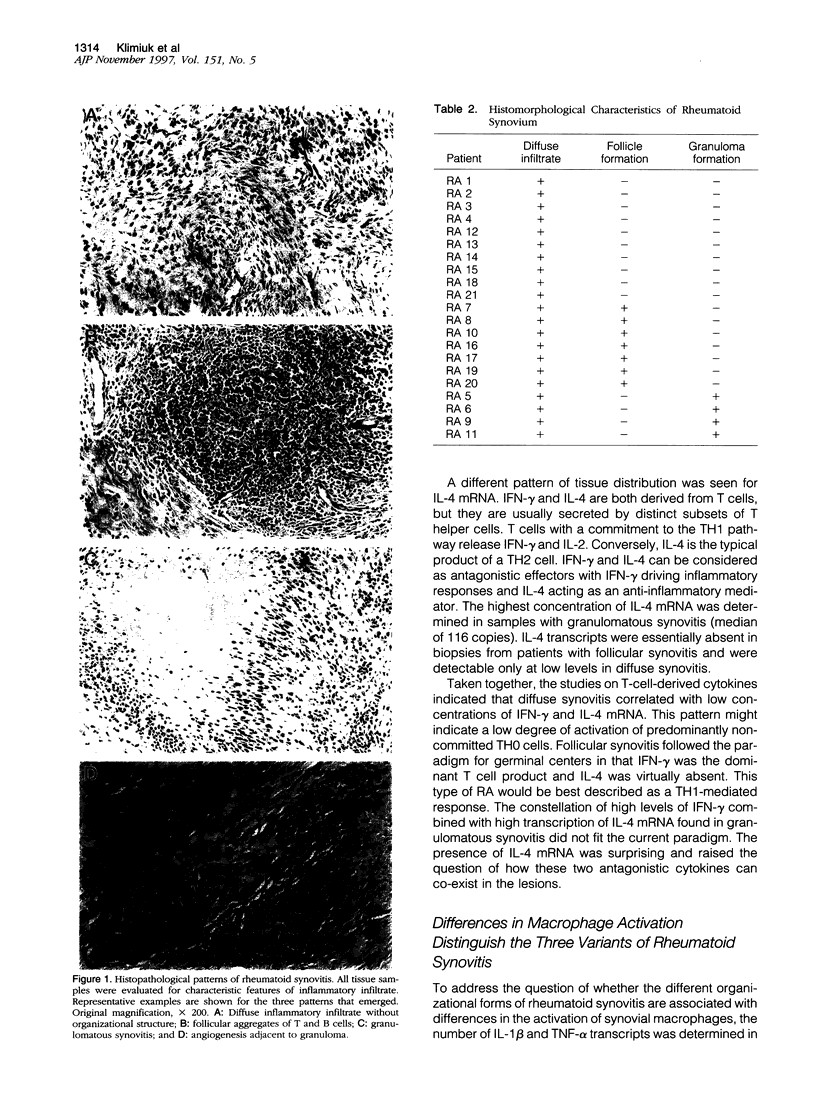
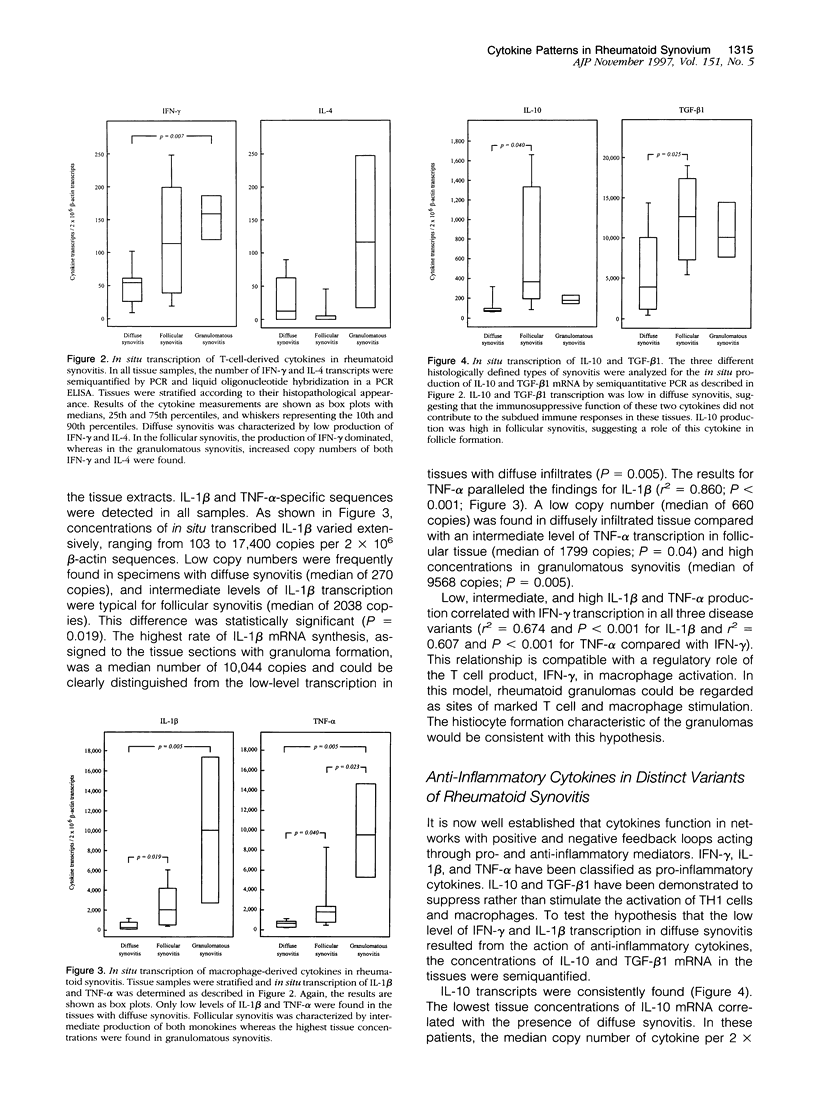
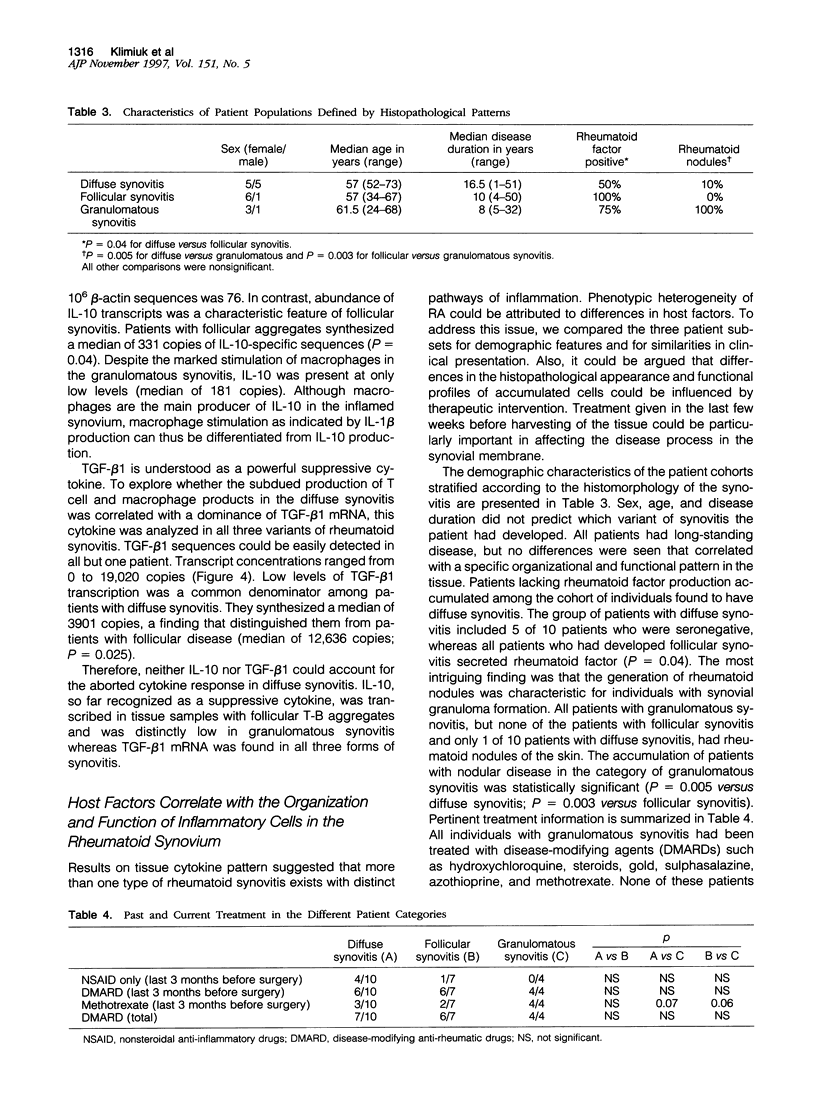



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Murphy K. M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996 Oct 31;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Boros D. L. The role of cytokines in the formation of the schistosome egg granuloma. Immunobiology. 1994 Oct;191(4-5):441–450. doi: 10.1016/S0171-2985(11)80450-X. [DOI] [PubMed] [Google Scholar]
- Brack A., Rittner H. L., Younge B. R., Kaltschmidt C., Weyand C. M., Goronzy J. J. Glucocorticoid-mediated repression of cytokine gene transcription in human arteritis-SCID chimeras. J Clin Invest. 1997 Jun 15;99(12):2842–2850. doi: 10.1172/JCI119477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Stuhlmüller B., Keyszer G., Kinne R. W. Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum. 1997 Jan;40(1):5–18. doi: 10.1002/art.1780400104. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Terebuh P. D., Warmington K. S., Hershey S. D., Evanoff H. L., Kunkel S. L., Higashi G. I. Role of IL-4 and IFN-gamma in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J Immunol. 1992 Feb 1;148(3):900–906. [PubMed] [Google Scholar]
- Chensue S. W., Warmington K. S., Hershey S. D., Terebuh P. D., Othman M., Kunkel S. L. Evolving T cell responses in murine schistosomiasis. Th2 cells mediate secondary granulomatous hypersensitivity and are regulated by CD8+ T cells in vivo. J Immunol. 1993 Aug 1;151(3):1391–1400. [PubMed] [Google Scholar]
- Chensue S. W., Warmington K., Ruth J., Lincoln P., Kuo M. C., Kunkel S. L. Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am J Pathol. 1994 Nov;145(5):1105–1113. [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Maini R. N. Rheumatoid arthritis. Cell. 1996 May 3;85(3):307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D. Relationships among antigen presentation, cytokines, immune deviation, and autoimmune disease. J Exp Med. 1995 Aug 1;182(2):279–282. doi: 10.1084/jem.182.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 1990 Jun;33(6):768–773. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- Fox D. A. The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis Rheum. 1997 Apr;40(4):598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- Goronzy J. J., Weyand C. M. T cells in rheumatoid arthritis. Paradigms and facts. Rheum Dis Clin North Am. 1995 Aug;21(3):655–674. [PubMed] [Google Scholar]
- Grzych J. M., Pearce E., Cheever A., Caulada Z. A., Caspar P., Heiny S., Lewis F., Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991 Feb 15;146(4):1322–1327. [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomaki P., Luukkainen R., Saario R., Toivanen P., Punnonen J. Interleukin-10 functions as an antiinflammatory cytokine in rheumatoid synovium. Arthritis Rheum. 1996 Mar;39(3):386–395. doi: 10.1002/art.1780390306. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. In situ studies of the germinal center reaction. Adv Immunol. 1995;60:267–288. doi: 10.1016/s0065-2776(08)60587-8. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. The germinal center: a crucible for lymphocyte selection. Semin Immunol. 1996 Jun;8(3):179–184. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Levy Y., Brouet J. C. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994 Jan;93(1):424–428. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R. Properties and functions of interleukin-10. Adv Immunol. 1994;56:1–26. [PubMed] [Google Scholar]
- Ollier W. E., MacGregor A. Genetic epidemiology of rheumatoid disease. Br Med Bull. 1995 Apr;51(2):267–285. doi: 10.1093/oxfordjournals.bmb.a072960. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Roberts A. D., Griffin J. P., Abrams J. S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993 Jul 1;151(1):518–525. [PubMed] [Google Scholar]
- Panayi G. S., Lanchbury J. S., Kingsley G. H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Perez L., Orte J., Brieva J. A. Terminal differentiation of spontaneous rheumatoid factor-secreting B cells from rheumatoid arthritis patients depends on endogenous interleukin-10. Arthritis Rheum. 1995 Dec;38(12):1771–1776. doi: 10.1002/art.1780381210. [DOI] [PubMed] [Google Scholar]
- Randen I., Mellbye O. J., Førre O., Natvig J. B. The identification of germinal centres and follicular dendritic cell networks in rheumatoid synovial tissue. Scand J Immunol. 1995 May;41(5):481–486. doi: 10.1111/j.1365-3083.1995.tb03596.x. [DOI] [PubMed] [Google Scholar]
- Schröder A. E., Greiner A., Seyfert C., Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Gazzinelli R., Sher A., Paul W. E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. K., Seipelt E., Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8562–8566. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S. J., Dighe A. S., Gubler U., Murphy K. M. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997 Mar 3;185(5):817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Walmsley M., Katsikis P. D., Abney E., Parry S., Williams R. O., Maini R. N., Feldmann M. Interleukin-10 inhibition of the progression of established collagen-induced arthritis. Arthritis Rheum. 1996 Mar;39(3):495–503. doi: 10.1002/art.1780390318. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Pathogenesis of rheumatoid arthritis. Med Clin North Am. 1997 Jan;81(1):29–55. doi: 10.1016/s0025-7125(05)70504-6. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Hicok K. C., Hunder G. G., Goronzy J. J. Tissue cytokine patterns in patients with polymyalgia rheumatica and giant cell arteritis. Ann Intern Med. 1994 Oct 1;121(7):484–491. doi: 10.7326/0003-4819-121-7-199410010-00003. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Tetzlaff N., Björnsson J., Brack A., Younge B., Goronzy J. J. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum. 1997 Jan;40(1):19–26. doi: 10.1002/art.1780400105. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Young C. L., Adamson T. C., 3rd, Vaughan J. H., Fox R. I. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1984 Jan;27(1):32–39. doi: 10.1002/art.1780270106. [DOI] [PubMed] [Google Scholar]
- van Roon J. A., van Roy J. L., Gmelig-Meyling F. H., Lafeber F. P., Bijlsma J. W. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis Rheum. 1996 May;39(5):829–835. doi: 10.1002/art.1780390516. [DOI] [PubMed] [Google Scholar]



