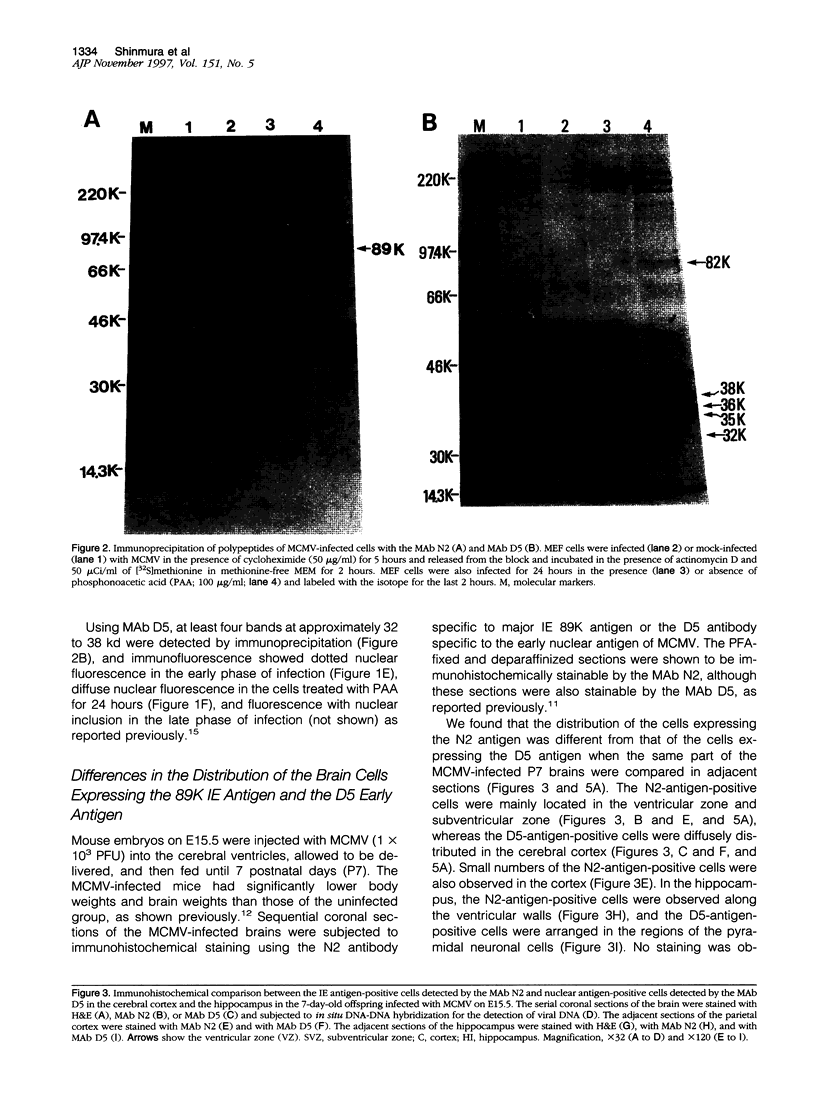
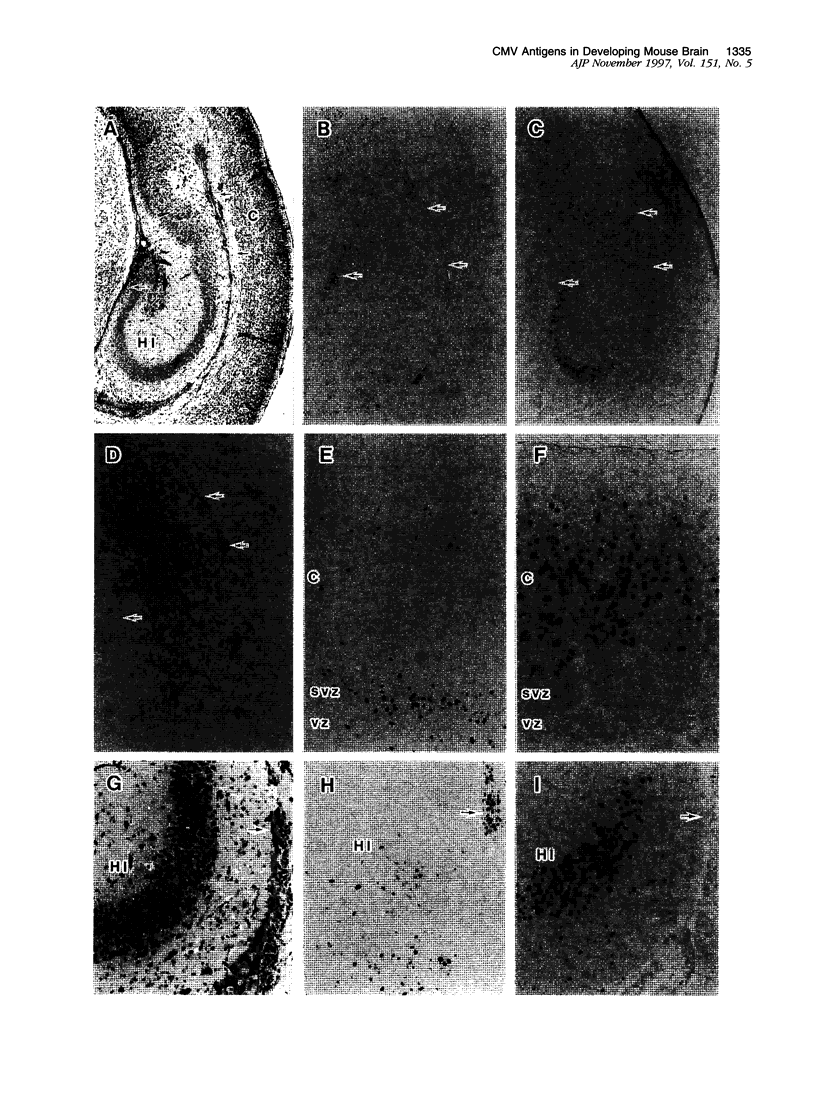
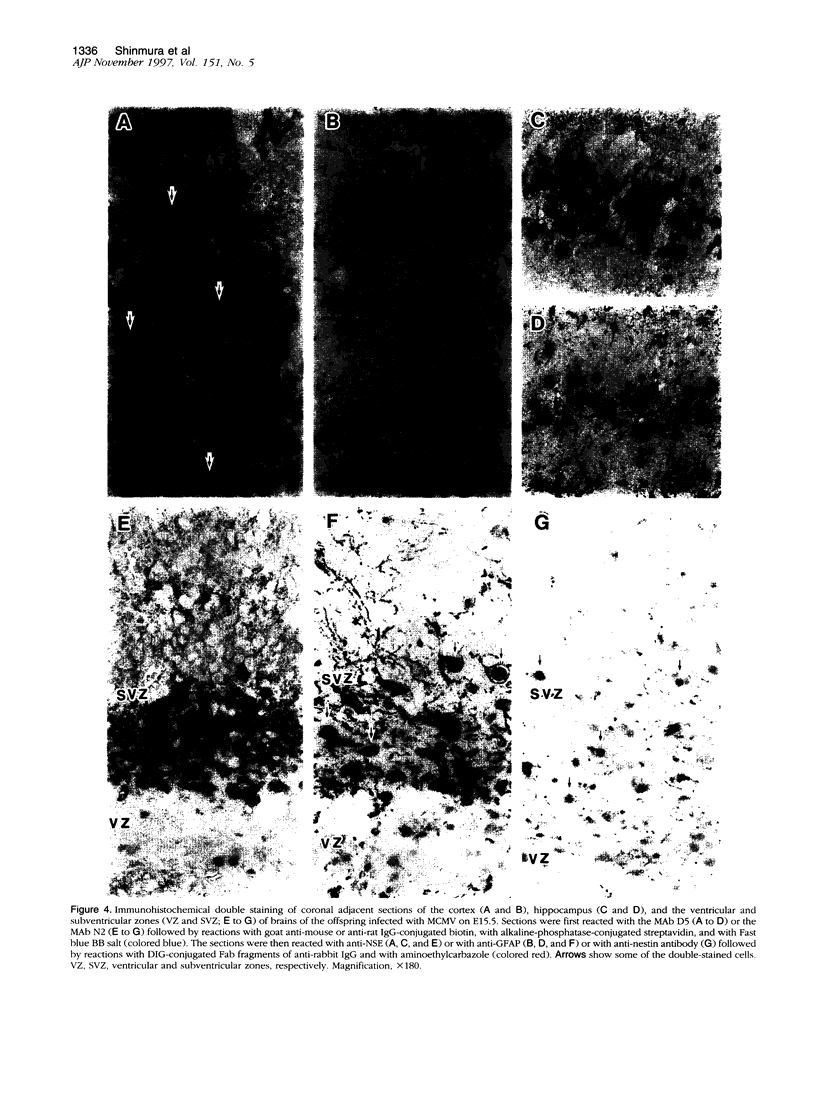
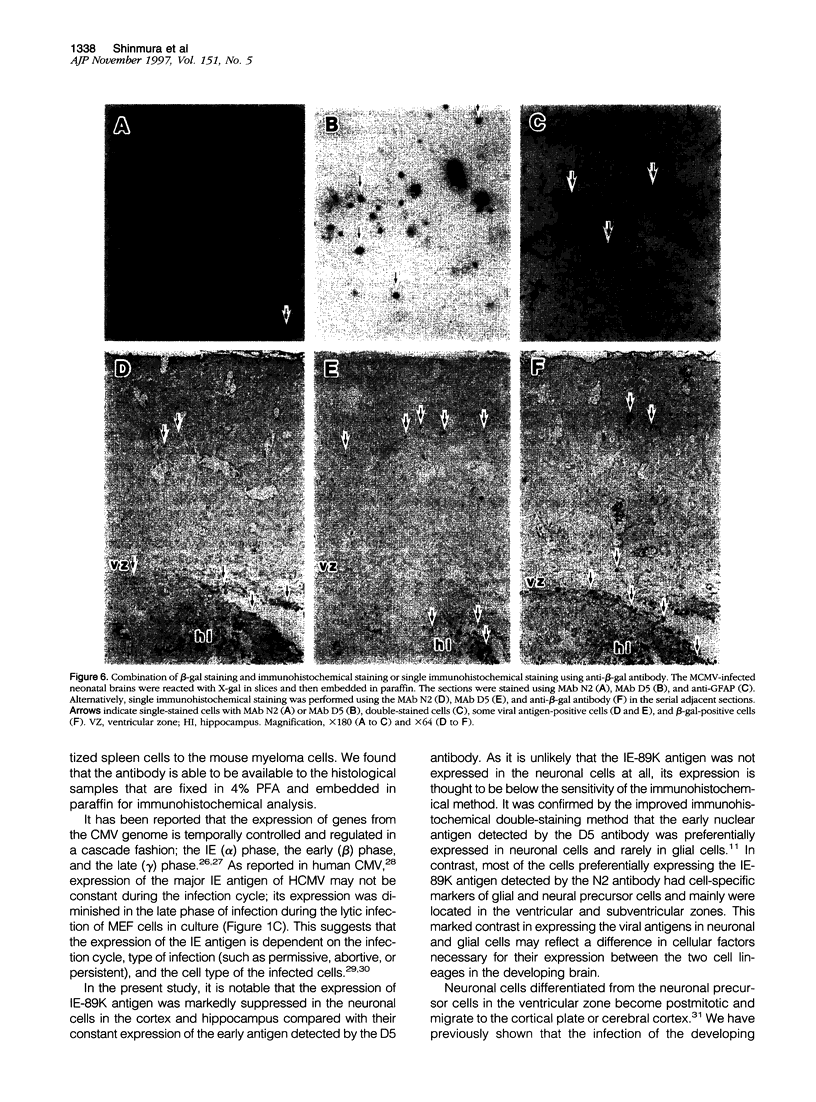
Abstract
Brain disorders induced by congenital cytomegalovirus (CMV) infection may appear at a later time after birth as a consequence of persistent infection and/or the activation of a latent infection of the neural cells. We have analyzed the infection dynamics of the neural cells in the neonatal mouse brains infected with murine CMV (MCMV) in the late stage of gestation. First we prepared a rat monoclonal antibody to the major immediate-early (IE)-89K antigen and then used the antibody for comparison of the expression of early and late viral genes in the developing mouse brains. The cells expressing the IE-89K antigen were mostly localized in the ventricular and subventricular zones and were preferentially double stained with anti-glial fibrillary acidic protein and anti-nestin antibodies. In contrast, the cells expressing the early nuclear antigen, detected by the monoclonal antibody D5, were diffusely distributed in the cortex and the hippocampus and were mostly double labeled with anti-neuron-specific enolase antibody. In neonatal mouse brains infected congenitally with recombinant MCMV, which expressed lacZ as a late gene, the number of the early nuclear antigen-positive cells was much higher than that of the beta-galactosidase-expressing cells, the number of which was almost the same as that of the IE-89K antigen-positive cells. In addition, the distribution of viral DNA-rich cells detected by DNA-DNA hybridization was similar to that of the IE-89K antigen-positive cells. These results suggest that CMV may persistently infect neuronal cells, whereas lytic infection may preferentially occur in the glial cells in the developing brain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale J. F., Jr Human cytomegalovirus infection and disorders of the nervous system. Arch Neurol. 1984 Mar;41(3):310–320. doi: 10.1001/archneur.1984.04050150092023. [DOI] [PubMed] [Google Scholar]
- Baskar J. F., Smith P. P., Ciment G. S., Hoffmann S., Tucker C., Tenney D. J., Colberg-Poley A. M., Nelson J. A., Ghazal P. Developmental analysis of the cytomegalovirus enhancer in transgenic animals. J Virol. 1996 May;70(5):3215–3226. doi: 10.1128/jvi.70.5.3215-3226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskar J. F., Smith P. P., Nilaver G., Jupp R. A., Hoffmann S., Peffer N. J., Tenney D. J., Colberg-Poley A. M., Ghazal P., Nelson J. A. The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice. J Virol. 1996 May;70(5):3207–3214. doi: 10.1128/jvi.70.5.3207-3214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton R. A., Tevethia M. J. Immunoprecipitation of virus-specific immediate-early and early polypeptides from cells lytically infected with human cytomegalovirus strain AD 169. Virology. 1981 Jul 15;112(1):262–273. doi: 10.1016/0042-6822(81)90631-0. [DOI] [PubMed] [Google Scholar]
- Conboy T. J., Pass R. F., Stagno S., Britt W. J., Alford C. A., McFarland C. E., Boll T. J. Intellectual development in school-aged children with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1986 Jun;77(6):801–806. [PubMed] [Google Scholar]
- Fritschy J. M., Brandner S., Aguzzi A., Koedood M., Luscher B., Mitchell P. J. Brain cell type specificity and gliosis-induced activation of the human cytomegalovirus immediate-early promoter in transgenic mice. J Neurosci. 1996 Apr 1;16(7):2275–2282. doi: 10.1523/JNEUROSCI.16-07-02275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P. D., Grundy J. E. Molecular biology and immunology of cytomegalovirus. Biochem J. 1987 Jan 15;241(2):313–324. doi: 10.1042/bj2410313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly E., Mucke L., Oldstone M. B. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991 Sep 13;253(5025):1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- Joly E., Oldstone M. B. Neuronal cells are deficient in loading peptides onto MHC class I molecules. Neuron. 1992 Jun;8(6):1185–1190. doi: 10.1016/0896-6273(92)90138-4. [DOI] [PubMed] [Google Scholar]
- Kashiwai A., Kawamura N., Kadota C., Tsutsui Y. Susceptibility of mouse embryo to murine cytomegalovirus infection in early and mid-gestation stages. Arch Virol. 1992;127(1-4):37–48. doi: 10.1007/BF01309573. [DOI] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate early times after infection. J Virol. 1984 Jun;50(3):784–795. doi: 10.1128/jvi.50.3.784-795.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Fibi M. R., Koszinowski U. H. Characterization of the major immediate-early polypeptides encoded by murine cytomegalovirus. J Virol. 1985 May;54(2):422–428. doi: 10.1128/jvi.54.2.422-428.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedood M., Fichtel A., Meier P., Mitchell P. J. Human cytomegalovirus (HCMV) immediate-early enhancer/promoter specificity during embryogenesis defines target tissues of congenital HCMV infection. J Virol. 1995 Apr;69(4):2194–2207. doi: 10.1128/jvi.69.4.2194-2207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U., Zimmerman L. B., McKay R. D. CNS stem cells express a new class of intermediate filament protein. Cell. 1990 Feb 23;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lubon H., Ghazal P., Hennighausen L., Reynolds-Kohler C., Lockshin C., Nelson J. Cell-specific activity of the modulator region in the human cytomegalovirus major immediate-early gene. Mol Cell Biol. 1989 Mar;9(3):1342–1345. doi: 10.1128/mcb.9.3.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Fields B. N., Dermody T. S. Prolonged replication in the mouse central nervous system of reoviruses isolated from persistently infected cell cultures. J Virol. 1993 Jun;67(6):3019–3026. doi: 10.1128/jvi.67.6.3019-3026.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse I., Tsutsui Y. Brain abnormalities induced by murine cytomegalovirus injected into the cerebral ventricles of mouse embryos exo utero. Teratology. 1989 Aug;40(2):181–189. doi: 10.1002/tera.1420400212. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Gnann J. W., Jr, Ghazal P. Regulation and tissue-specific expression of human cytomegalovirus. Curr Top Microbiol Immunol. 1990;154:75–100. doi: 10.1007/978-3-642-74980-3_4. [DOI] [PubMed] [Google Scholar]
- Pass R. F., Stagno S., Myers G. J., Alford C. A. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics. 1980 Nov;66(5):758–762. [PubMed] [Google Scholar]
- Poland S. D., Costello P., Dekaban G. A., Rice G. P. Cytomegalovirus in the brain: in vitro infection of human brain-derived cells. J Infect Dis. 1990 Dec;162(6):1252–1262. doi: 10.1093/infdis/162.6.1252. [DOI] [PubMed] [Google Scholar]
- Pomeroy C., Hilleren P. J., Jordan M. C. Latent murine cytomegalovirus DNA in splenic stromal cells of mice. J Virol. 1991 Jun;65(6):3330–3334. doi: 10.1128/jvi.65.6.3330-3334.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988 Jul 8;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Reynolds K., Mezey E., Zimmer A. Activity of the beta-retinoic acid receptor promoter in transgenic mice. Mech Dev. 1991 Dec;36(1-2):15–29. doi: 10.1016/0925-4773(91)90068-h. [DOI] [PubMed] [Google Scholar]
- Shering A. F., Bain D., Stewart K., Epstein A. L., Castro M. G., Wilkinson G. W., Lowenstein P. R. Cell type-specific expression in brain cell cultures from a short human cytomegalovirus major immediate early promoter depends on whether it is inserted into herpesvirus or adenovirus vectors. J Gen Virol. 1997 Feb;78(Pt 2):445–459. doi: 10.1099/0022-1317-78-2-445. [DOI] [PubMed] [Google Scholar]
- Shinmura Y., Kosugi I., Aiba-Masago S., Baba S., Yong L. R., Tsutsui Y. Disordered migration and loss of virus-infected neuronal cells in developing mouse brains infected with murine cytomegalovirus. Acta Neuropathol. 1997 Jun;93(6):551–557. doi: 10.1007/s004010050651. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Stagno S., Pass R. F., Cloud G., Britt W. J., Henderson R. E., Walton P. D., Veren D. A., Page F., Alford C. A. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986 Oct 10;256(14):1904–1908. [PubMed] [Google Scholar]
- Stamminger T., Fickenscher H., Fleckenstein B. Cell type-specific induction of the major immediate early enhancer of human cytomegalovirus by cyclic AMP. J Gen Virol. 1990 Jan;71(Pt 1):105–113. doi: 10.1099/0022-1317-71-1-105. [DOI] [PubMed] [Google Scholar]
- Stoddart C. A., Cardin R. D., Boname J. M., Manning W. C., Abenes G. B., Mocarski E. S. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol. 1994 Oct;68(10):6243–6253. doi: 10.1128/jvi.68.10.6243-6253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishon A., Eddleston M., de la Torre J. C., Oldstone M. B. Cytotoxic T lymphocytes cleanse viral gene products from individually infected neurons and lymphocytes in mice persistently infected with lymphocytic choriomeningitis virus. Virology. 1993 Nov;197(1):463–467. doi: 10.1006/viro.1993.1613. [DOI] [PubMed] [Google Scholar]
- Tomooka Y., Kitani H., Jing N., Matsushima M., Sakakura T. Reconstruction of neural tube-like structures in vitro from primary neural precursor cells. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9683–9687. doi: 10.1073/pnas.90.20.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui Y., Kashiwai A., Kawamura N., Aiba-Masago S., Kosugi I. Prolonged infection of mouse brain neurons with murine cytomegalovirus after pre- and perinatal infection. Arch Virol. 1995;140(10):1725–1736. doi: 10.1007/BF01384337. [DOI] [PubMed] [Google Scholar]
- Tsutsui Y., Kashiwai A., Kawamura N., Kadota C. Microphthalmia and cerebral atrophy induced in mouse embryos by infection with murine cytomegalovirus in midgestation. Am J Pathol. 1993 Sep;143(3):804–813. [PMC free article] [PubMed] [Google Scholar]
- Tsutsui Y., Kashiwai A., Kawamura N., Nagahama M., Mizutani A., Naruse I. Susceptibility of brain cells to murine cytomegalovirus infection in the developing mouse brain. Acta Neuropathol. 1989;79(3):262–270. doi: 10.1007/BF00294660. [DOI] [PubMed] [Google Scholar]
- Tsutsui Y., Naruse I. Murine cytomegalovirus infection of cultured mouse embryos. Am J Pathol. 1987 May;127(2):262–270. [PMC free article] [PubMed] [Google Scholar]
- Tsutsui Y., Nogami-Satake T. Differential expression of the major immediate early gene of human cytomegalovirus. J Gen Virol. 1990 Jan;71(Pt 1):115–124. doi: 10.1099/0022-1317-71-1-115. [DOI] [PubMed] [Google Scholar]
- Tsutsui Y., Yamazaki Y. Subcellular distribution of the major immediate early proteins of human cytomegalovirus changes during infection. J Gen Virol. 1991 Jun;72(Pt 6):1415–1419. doi: 10.1099/0022-1317-72-6-1415. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N., Kors N., Van Nieuwmegen R. Double immunocytochemical staining in the study of antibody-producing cells in vivo. Detection of specific antibody-producing cells in the spleen and simultaneous determination whether or not they produce immunoglobulin G antibodies. J Histochem Cytochem. 1984 Jul;32(7):677–680. doi: 10.1177/32.7.6376616. [DOI] [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971 Jul 22;285(4):203–214. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]