Abstract
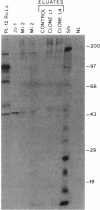
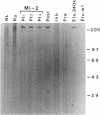
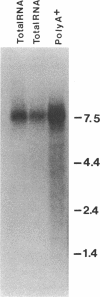
Anti-Mi-2 autoantibody is strongly associated with dermatomyositis and found in sera of 20% of patients. Mi-2 antigen contains at least eight components and previous evidence suggested that the 240-kD protein was the antigenic component for at least some sera. In this study, anti-M-2 patient sera were used to screen human thymocyte and HeLa cell lambda gt11 expression libraries, and two clones from each had plaques specifically reactive with anti-Mi-2 sera. Studies with affinity-purified antibody supported the identification of the clones. All of 44 anti-Mi-2 sera reacted with the plaques, but none of 44 control sera reacted significantly. The cDNAs were identical, and full sequencing of one revealed an open reading frame spanning a 1,054-bp insert. Rescreening the library with the cDNA yielded a 1,589-bp cDNA that continued the open reading frame. The Mi-2 cDNA hybridized to a single 7.5-8.0 kb mRNA of HeLa cells, by Northern blot. Rabbit antiserum directed at a portion of the cDNA product reacted with HeLa 240-kD Mi-2 protein. The sequence was notable for four potential zinc-fingers and several charged regions. The protein encoded by the cDNA produced in vitro reacted with only one of five of the Mi-2 sera. These findings indicate that the Mi-2 240 kD is a novel protein that is antigenic for all Mi-2 sera, and strongly suggests that a major common epitope is conformational in nature.
Full text
PDF

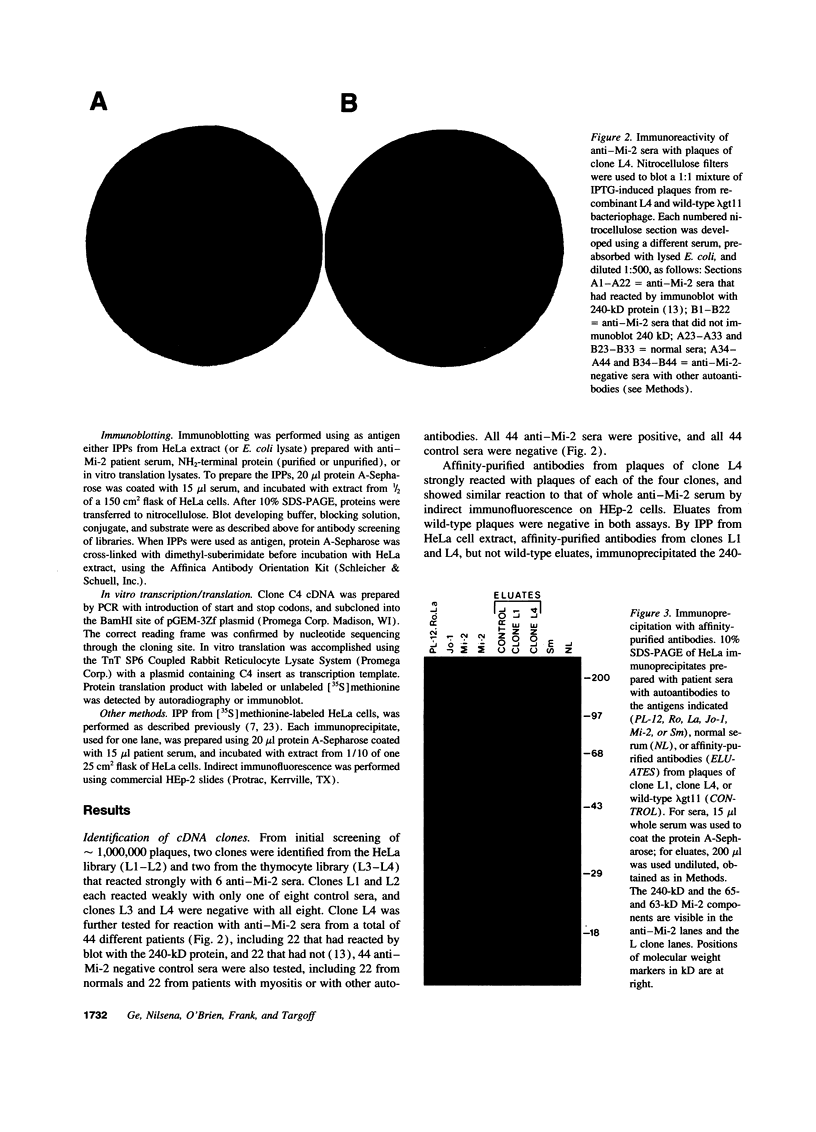





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Kerlavage A. R., Fields C., Venter J. C. 3,400 new expressed sequence tags identify diversity of transcripts in human brain. Nat Genet. 1993 Jul;4(3):256–267. doi: 10.1038/ng0793-256. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Brendel V., Bucher P., Nourbakhsh I. R., Blaisdell B. E., Karlin S. Methods and algorithms for statistical analysis of protein sequences. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2002–2006. doi: 10.1073/pnas.89.6.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel V., Dohlman J., Blaisdell B. E., Karlin S. Very long charge runs in systemic lupus erythematosus-associated autoantigens. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1536–1540. doi: 10.1073/pnas.88.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. C., Bernstein R. M., Mathews M. B. Autoantibodies against alanyl-tRNA synthetase and tRNAAla coexist and are associated with myositis. J Exp Med. 1986 May 1;163(5):1281–1291. doi: 10.1084/jem.163.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ge Q., Frank M. B., O'Brien C., Targoff I. N. Cloning of a complementary DNA coding for the 100-kD antigenic protein of the PM-Scl autoantigen. J Clin Invest. 1992 Aug;90(2):559–570. doi: 10.1172/JCI115895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Love L. A., Leff R. L., Fraser D. D., Targoff I. N., Dalakas M., Plotz P. H., Miller F. W. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991 Nov;70(6):360–374. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- Ma J., Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987 Mar 13;48(5):847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature. 1983 Jul 14;304(5922):177–179. doi: 10.1038/304177a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Reichlin M., Hughes G. R., Bernstein R. M. Anti-threonyl-tRNA synthetase, a second myositis-related autoantibody. J Exp Med. 1984 Aug 1;160(2):420–434. doi: 10.1084/jem.160.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister G., Roby-Shemkovitz A., Amara S. G., Lerner M. R. cDNA sequence of the rat U snRNP-associated protein N: description of a potential Sm epitope. EMBO J. 1989 Apr;8(4):1177–1181. doi: 10.1002/j.1460-2075.1989.tb03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilasena D. S., Trieu E. P., Targoff I. N. Analysis of the Mi-2 autoantigen of dermatomyositis. Arthritis Rheum. 1995 Jan;38(1):123–128. doi: 10.1002/art.1780380119. [DOI] [PubMed] [Google Scholar]
- Nishikai M., Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum. 1980 Aug;23(8):881–888. doi: 10.1002/art.1780230802. [DOI] [PubMed] [Google Scholar]
- Ohosone Y., Mimori T., Griffith A., Akizuki M., Homma M., Craft J., Hardin J. A. Molecular cloning of cDNA encoding Sm autoantigen: derivation of a cDNA for a B polypeptide of the U series of small nuclear ribonucleoprotein particles. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4249–4253. doi: 10.1073/pnas.86.11.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider L. G., Miller F. W., Targoff I. N., Sherry D. D., Samayoa E., Lindahl M., Wener M. H., Pachman L. M., Plotz P. H. A broadened spectrum of juvenile myositis. Myositis-specific autoantibodies in children. Arthritis Rheum. 1994 Oct;37(10):1534–1538. doi: 10.1002/art.1780371019. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Rokeach L. A., Haselby J. A., Hoch S. O. Molecular cloning of a cDNA encoding the human Sm-D autoantigen. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4832–4836. doi: 10.1073/pnas.85.13.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff L. A., Nibert M. L., Co M. S., Brown E. G., Fields B. N. Distinct binding sites for zinc and double-stranded RNA in the reovirus outer capsid protein sigma 3. Mol Cell Biol. 1988 Jan;8(1):273–283. doi: 10.1128/mcb.8.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler P. B. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988 May 19;333(6170):210–212. doi: 10.1038/333210a0. [DOI] [PubMed] [Google Scholar]
- Targoff I. N. Autoantibodies in polymyositis. Rheum Dis Clin North Am. 1992 May;18(2):455–482. [PubMed] [Google Scholar]
- Targoff I. N. Autoantibodies to aminoacyl-transfer RNA synthetases for isoleucine and glycine. Two additional synthetases are antigenic in myositis. J Immunol. 1990 Mar 1;144(5):1737–1743. [PubMed] [Google Scholar]
- Targoff I. N. Humoral immunity in polymyositis/dermatomyositis. J Invest Dermatol. 1993 Jan;100(1):116S–123S. doi: 10.1111/1523-1747.ep12356607. [DOI] [PubMed] [Google Scholar]
- Targoff I. N., Johnson A. E., Miller F. W. Antibody to signal recognition particle in polymyositis. Arthritis Rheum. 1990 Sep;33(9):1361–1370. doi: 10.1002/art.1780330908. [DOI] [PubMed] [Google Scholar]
- Targoff I. N., Reichlin M. The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum. 1985 Jul;28(7):796–803. doi: 10.1002/art.1780280711. [DOI] [PubMed] [Google Scholar]
- Targoff I. N., Trieu E. P., Miller F. W. Reaction of anti-OJ autoantibodies with components of the multi-enzyme complex of aminoacyl-tRNA synthetases in addition to isoleucyl-tRNA synthetase. J Clin Invest. 1993 Jun;91(6):2556–2564. doi: 10.1172/JCI116493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]