Abstract
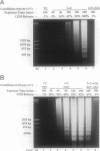
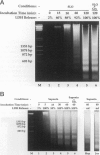
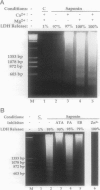
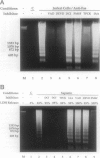
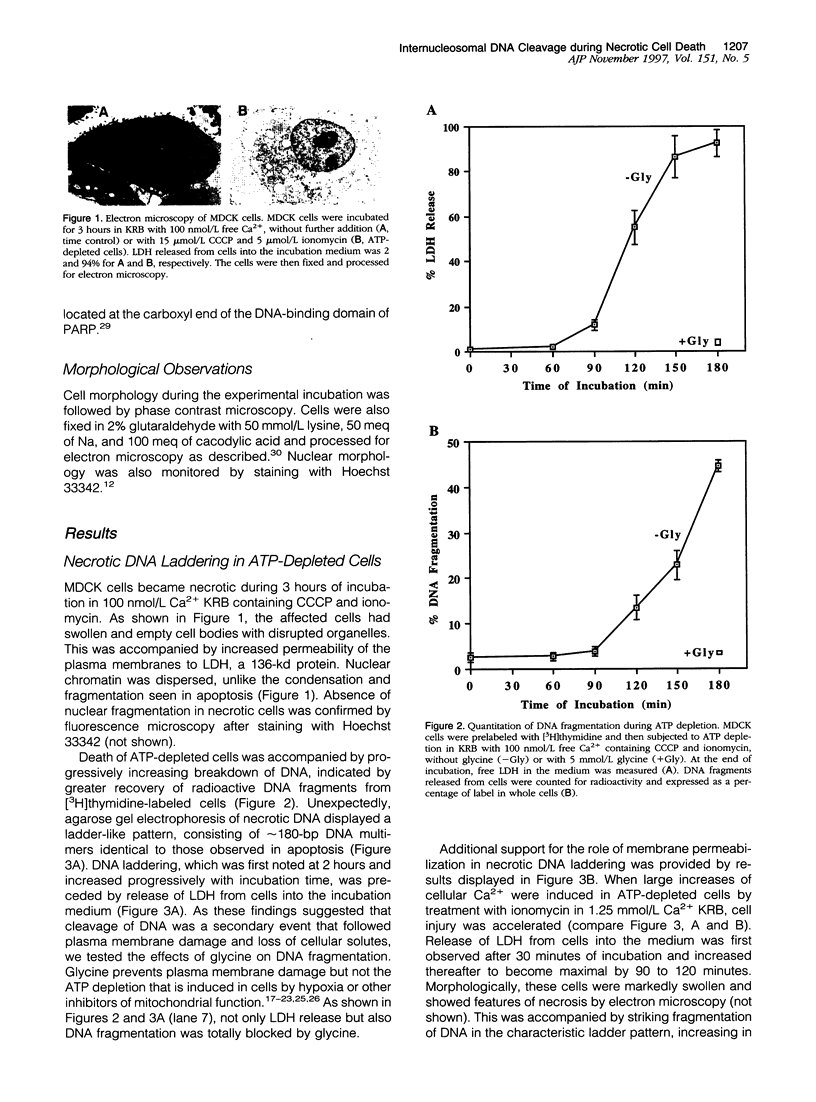
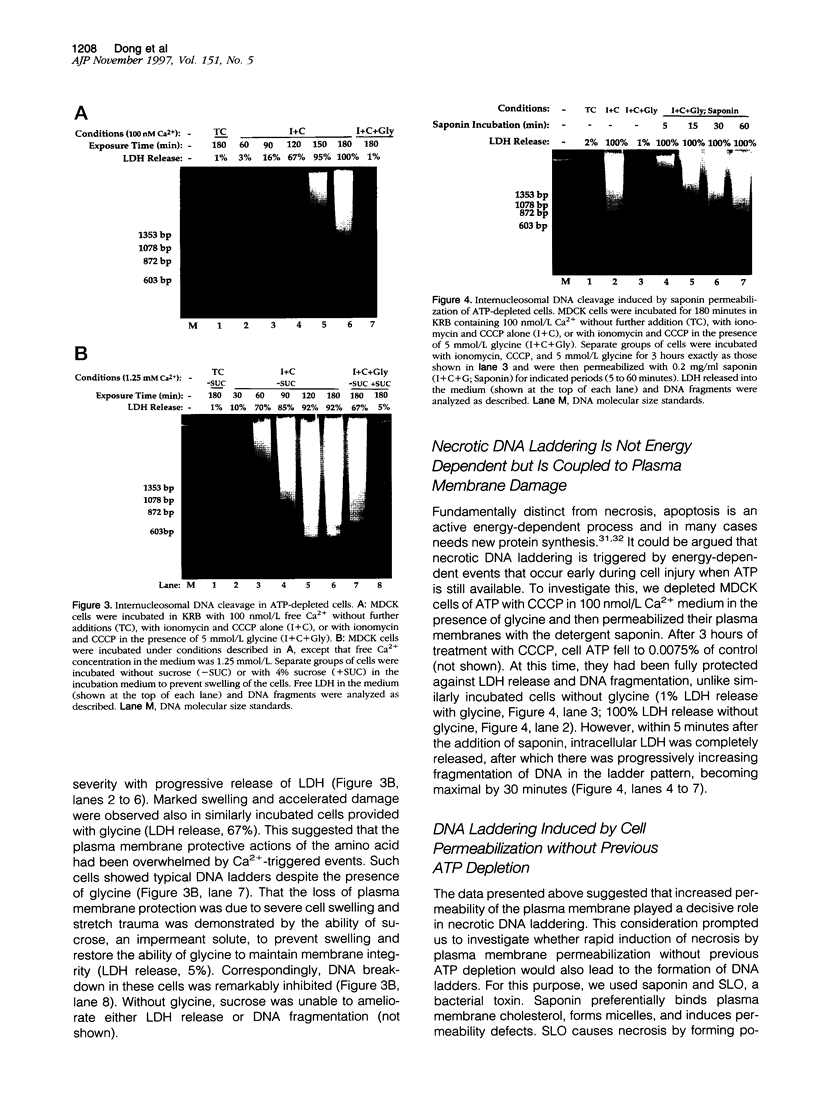
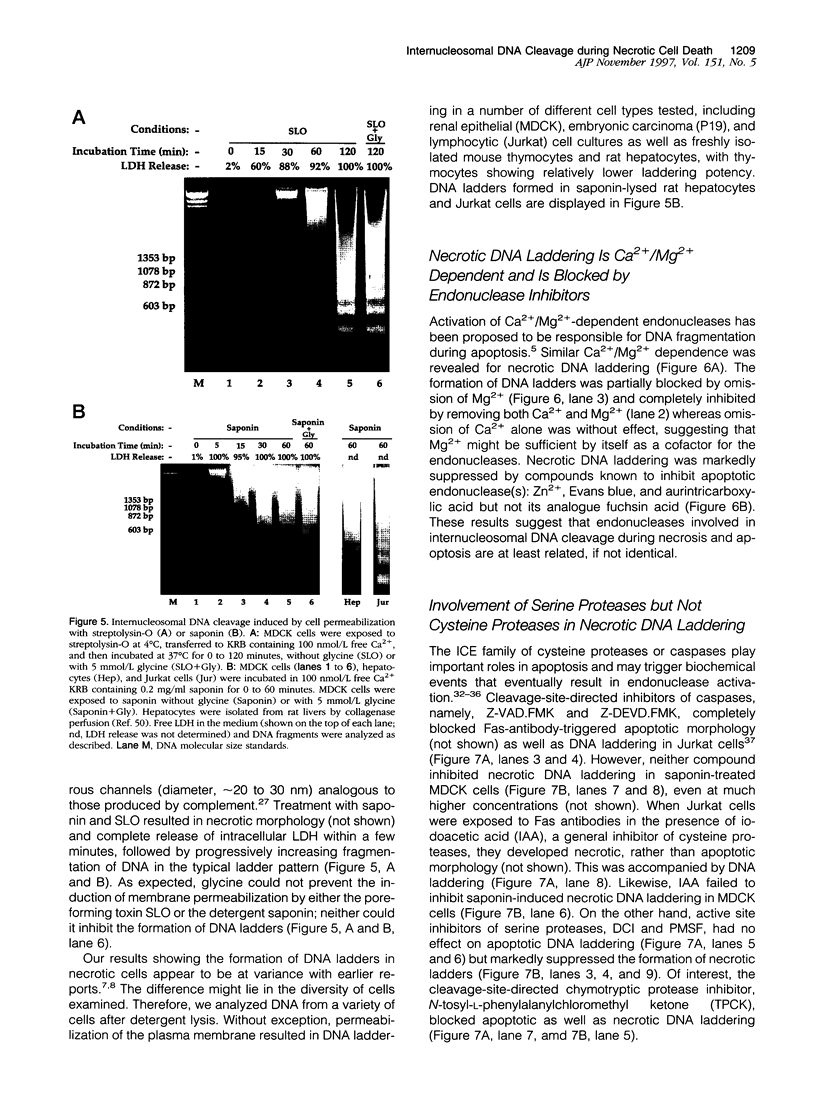
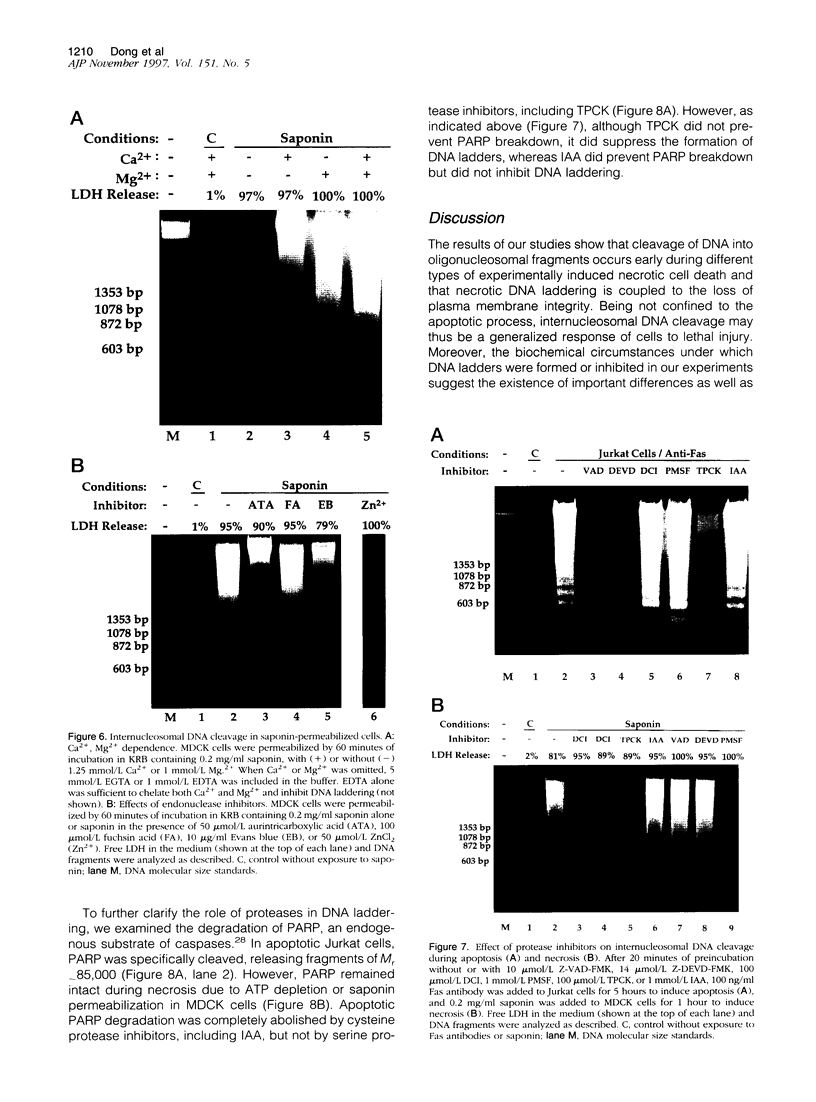
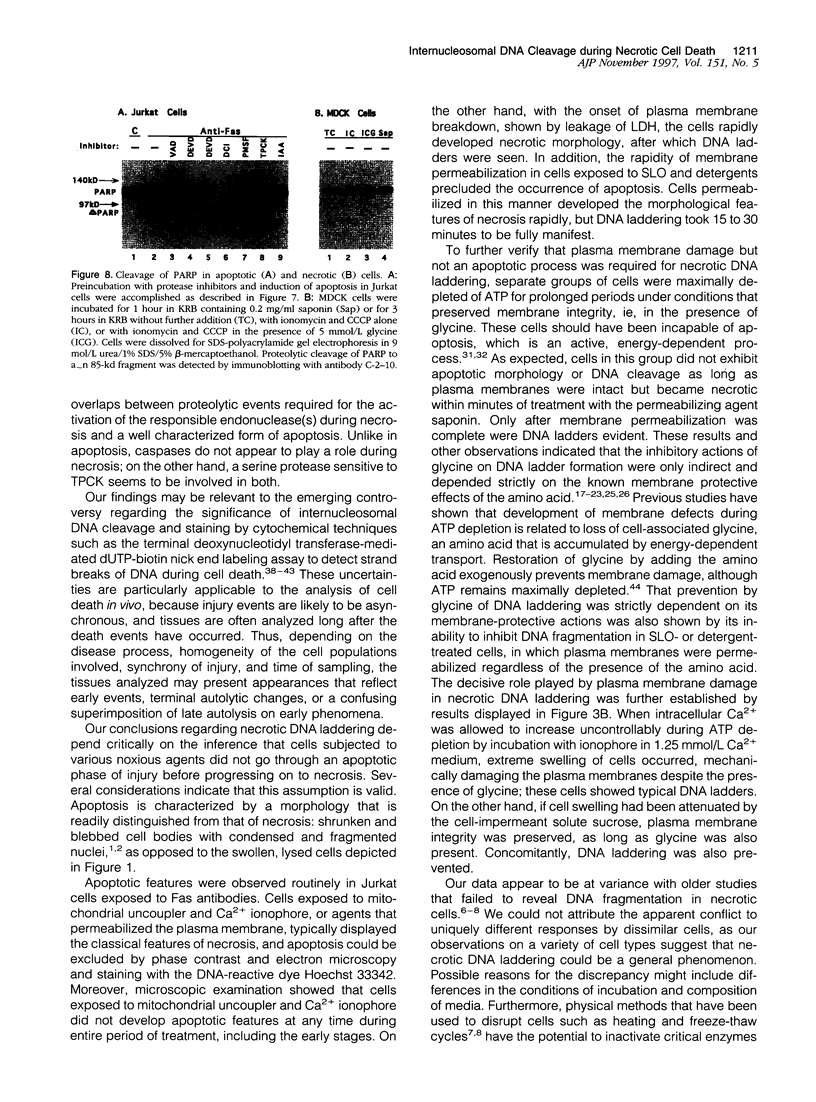
Autolytic DNA breakdown, detected as smears in electrophoretic gels, is a late event in necrosis. On the other hand, internucleosomal DNA cleavage, visualized as ladders, is thought to be a hallmark of apoptosis. We now report that this specific form of DNA fragmentation also occurs during necrosis and is an early event but appears to be triggered by proteolytic mechanisms significantly different from those documented in apoptosis. Treatment of MDCK cells with a mitochondrial uncoupler and a Ca2+ ionophore led to ATP depletion, necrotic morphology, and progressive fragmentation of DNA in an internucleosomal or ladder pattern. DNA breakdown was immediately preceded by increased permeability of the plasma membrane to macromolecules. Provision of glycine along with the noxious agents did not modify the extent of ATP depletion, but prevented plasma membrane damage. This was accompanied by complete inhibition of DNA fragmentation. Internucleosomal DNA cleavage was observed also during necrosis after rapid permeabilization of plasma membranes by detergents or streptolysin-O in hepatocytes, thymocytes, and P19, Jurkat, and MDCK cells. DNA fragmentation associated with necrosis was Ca2+/Mg2+ dependent, was suppressed by endonuclease inhibitors, and was abolished by serine protease inhibitors but not by inhibitors of interleukin-1 beta converting enzyme (ICE)-related proteases or caspases. Moreover, unlike apoptosis, it was not accompanied by caspase-mediated proteolysis. On the other hand, the cleavage-site-directed chymotryptic inhibitor N-tosyl-L-phenylalanyl-chloromethyl ketone (TPCK) suppressed DNA fragmentation not only in necrotic cells but also during Fas-mediated apoptosis, without inhibiting caspase-related proteolysis. The results suggest a novel pathway of endonuclease activation during necrosis not involving the participation of caspases. In addition, they indicate that techniques based on double-strand DNA breaks may not reliably differentiate between apoptosis and necrosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Livingston D. J., Nicholson D. W., Salvesen G., Thornberry N. A., Wong W. W., Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996 Oct 18;87(2):171–171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Bortner C. D., Oldenburg N. B., Cidlowski J. A. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995 Jan;5(1):21–26. doi: 10.1016/s0962-8924(00)88932-1. [DOI] [PubMed] [Google Scholar]
- Boyles J., Fox J. E., Phillips D. R., Stenberg P. E. Organization of the cytoskeleton in resting, discoid platelets: preservation of actin filaments by a modified fixation that prevents osmium damage. J Cell Biol. 1985 Oct;101(4):1463–1472. doi: 10.1083/jcb.101.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch W., Oberhammer F., Schulte-Hermann R. Cell death by apoptosis and its protective role against disease. Trends Pharmacol Sci. 1992 Jun;13(6):245–251. doi: 10.1016/0165-6147(92)90077-j. [DOI] [PubMed] [Google Scholar]
- Cohen G. M., Sun X. M., Snowden R. T., Dinsdale D., Skilleter D. N. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1992 Sep 1;286(Pt 2):331–334. doi: 10.1042/bj2860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Collins R. J., Harmon B. V., Gobé G. C., Kerr J. F. Internucleosomal DNA cleavage should not be the sole criterion for identifying apoptosis. Int J Radiat Biol. 1992 Apr;61(4):451–453. doi: 10.1080/09553009214551201. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Bronk S. F., Gores G. J. Glycine cytoprotection during lethal hepatocellular injury from adenosine triphosphate depletion. Gastroenterology. 1992 Jun;102(6):2098–2107. doi: 10.1016/0016-5085(92)90338-y. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995 Jun;7(3):337–343. doi: 10.1016/0955-0674(95)80088-3. [DOI] [PubMed] [Google Scholar]
- Enright H., Hebbel R. P., Nath K. A. Internucleosomal cleavage of DNA as the sole criterion for apoptosis may be artifactual. J Lab Clin Med. 1994 Jul;124(1):63–68. [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Garza-Quintero R., Weinberg J. M., Ortega-Lopez J., Davis J. A., Venkatachalam M. A. Conservation of structure in ATP-depleted proximal tubules: role of calcium, polyphosphoinositides, and glycine. Am J Physiol. 1993 Nov;265(5 Pt 2):F605–F623. doi: 10.1152/ajprenal.1993.265.5.F605. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B., Ruttkay-Nedecky B., Koudelka H., Bukowska K., Bursch W., Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995 May;21(5):1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- Hegyi L., Hardwick S. J., Mitchinson M. J., Skepper J. N. The presence of apoptotic cells in human atherosclerotic lesions. Am J Pathol. 1997 Jan;150(1):371–373. [PMC free article] [PubMed] [Google Scholar]
- Iwata M., Myerson D., Torok-Storb B., Zager R. A. An evaluation of renal tubular DNA laddering in response to oxygen deprivation and oxidant injury. J Am Soc Nephrol. 1994 Dec;5(6):1307–1313. doi: 10.1681/ASN.V561307. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Desnoyers S., Ottaviano Y., Davidson N. E., Poirier G. G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993 Sep 1;53(17):3976–3985. [PubMed] [Google Scholar]
- Kumar S. ICE-like proteases in apoptosis. Trends Biochem Sci. 1995 May;20(5):198–202. doi: 10.1016/s0968-0004(00)89007-6. [DOI] [PubMed] [Google Scholar]
- Lamarre D., Talbot B., de Murcia G., Laplante C., Leduc Y., Mazen A., Poirier G. G. Structural and functional analysis of poly(ADP ribose) polymerase: an immunological study. Biochim Biophys Acta. 1988 Jul 13;950(2):147–160. doi: 10.1016/0167-4781(88)90007-3. [DOI] [PubMed] [Google Scholar]
- Lizard G., Fournel S., Genestier L., Dhedin N., Chaput C., Flacher M., Mutin M., Panaye G., Revillard J. P. Kinetics of plasma membrane and mitochondrial alterations in cells undergoing apoptosis. Cytometry. 1995 Nov 1;21(3):275–283. doi: 10.1002/cyto.990210308. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Schnellmann R. G., Jacobs W. R. Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest. 1990 Feb;85(2):316–324. doi: 10.1172/JCI114440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino M. J., Murphy M. K., Grabau G. G., Anderson C. B. Protective effects of glycine during hypothermic renal ischemia-reperfusion injury. Am J Physiol. 1991 Nov;261(5 Pt 2):F841–F848. doi: 10.1152/ajprenal.1991.261.5.F841. [DOI] [PubMed] [Google Scholar]
- Matsui H., Tsuji S., Nishimura H., Nagasawa S. Activation of the alternative pathway of complement by apoptotic Jurkat cells. FEBS Lett. 1994 Sep 12;351(3):419–422. doi: 10.1016/0014-5793(94)00897-3. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Moore H. P. Reconstitution of constitutive secretion using semi-intact cells: regulation by GTP but not calcium. J Cell Biol. 1991 Jan;112(1):39–54. doi: 10.1083/jcb.112.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundle S. D., Raza A. The two in situ techniques do not differentiate between apoptosis and necrosis but rather reveal distinct patterns of DNA fragmentation in apoptosis. Lab Invest. 1995 May;72(5):611–613. [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997 Feb 7;88(3):355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod M. G., Sun X. M., Snowden R. T., Davies R., Fearnhead H., Cohen G. M. Increased membrane permeability of apoptotic thymocytes: a flow cytometric study. Cytometry. 1993;14(6):595–602. doi: 10.1002/cyto.990140603. [DOI] [PubMed] [Google Scholar]
- Russell J. H., Dobos C. B. Mechanisms of immune lysis. II. CTL-induced nuclear disintegration of the target begins within minutes of cell contact. J Immunol. 1980 Sep;125(3):1256–1261. [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Bishop C. J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;17(Pt 2):229–259. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Silva P., Rosen S., Spokes K., Epstein F. H. Effect of glycine on medullary thick ascending limb injury in perfused kidneys. Kidney Int. 1991 Apr;39(4):653–658. doi: 10.1038/ki.1991.78. [DOI] [PubMed] [Google Scholar]
- Slee E. A., Zhu H., Chow S. C., MacFarlane M., Nicholson D. W., Cohen G. M. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 1996 Apr 1;315(Pt 1):21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Obermiller P. S., Eckhart W., Apgar J. R., Berger N. A., Meyers J. Genome digestion is a dispensable consequence of physiological cell death mediated by cytotoxic T lymphocytes. Mol Cell Biol. 1992 Jul;12(7):3060–3069. doi: 10.1128/mcb.12.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N., Walker P. D., Hsu S. M., Shah S. V. Activation of a 15-kDa endonuclease in hypoxia/reoxygenation injury without morphologic features of apoptosis. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7202–7206. doi: 10.1073/pnas.92.16.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. L., Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Weinberg J. M., Patel Y., Hussong U., Davis J. A. Effects of Ca++ and glycine on lipid breakdown and death of ATP-depleted MDCK cells. Kidney Int. 1995 Jul;48(1):118–128. doi: 10.1038/ki.1995.275. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Roeser N. F., Venkatachalam M. A. Role of increased cytosolic free calcium in the pathogenesis of rabbit proximal tubule cell injury and protection by glycine or acidosis. J Clin Invest. 1991 Feb;87(2):581–590. doi: 10.1172/JCI115033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M., Nissim I., Roeser N. F., Davis J. A., Schultz S., Nissim I. Relationships between intracellular amino acid levels and protection against injury to isolated proximal tubules. Am J Physiol. 1991 Mar;260(3 Pt 2):F410–F419. doi: 10.1152/ajprenal.1991.260.3.F410. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Venkatachalam M. A., Garzo-Quintero R., Roeser N. F., Davis J. A. Structural requirements for protection by small amino acids against hypoxic injury in kidney proximal tubules. FASEB J. 1990 Dec;4(15):3347–3354. doi: 10.1096/fasebj.4.15.2253849. [DOI] [PubMed] [Google Scholar]
- Wright S. C., Wei Q. S., Zhong J., Zheng H., Kinder D. H., Larrick J. W. Purification of a 24-kD protease from apoptotic tumor cells that activates DNA fragmentation. J Exp Med. 1994 Dec 1;180(6):2113–2123. doi: 10.1084/jem.180.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Death from inside out: an overview. Philos Trans R Soc Lond B Biol Sci. 1994 Aug 30;345(1313):237–241. doi: 10.1098/rstb.1994.0099. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]