Abstract
Cultured cerebellar granule neurons are widely used as a cellular model to study mechanisms of neuronal cell death because they undergo programmed cell death when switched from a culture medium containing 25 mM to one containing 5 mM K+. We have found that the growth-related gene product β (GROβ) partially prevents the K+-depletion-induced cell death, and that the neuroprotective action of GROβ on granule cells is mediated through the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) type of ionotropic glutamate receptors. GROβ-induced survival was suppressed by 6-cyano-7-nitroquinoxaline-2,3-dione, which is a specific antagonist of AMPA/kainate receptors; it was not affected by the inhibitor of N-methyl-d-aspartate receptors, 2-amino-5-phosphonopentanoic acid, and was comparable to the survival of granule cells induced by AMPA (10 μM) treatment. Moreover, GROβ-induced neuroprotection was abolished when granule cells were treated with antisense oligonucleotides specific for the AMPA receptor subunits, which significantly reduced receptor expression, as verified by Western blot analysis with subunit-specific antibodies and by granule cell electrophysiological sensitivity to AMPA. Our data demonstrate that GROβ is neurotrophic for cerebellar granule cells, and that this activity depends on AMPA receptors.
Keywords: chemokines, apoptosis, cerebellar granule neurons, glutamate receptors
The chemokine growth-related gene product β (GROβ) is a small pro-inflammatory cytokine with chemoattractant and regulatory activities toward inflammatory cells (1). GROβ belongs to the CXC chemokine family, which is characterized by the presence of a single amino acid (X) in a conserved cystein motif and which shares the same receptor with interleukin 8 (IL-8), IL-8RB/CXCR2. Chemokines of the CXC family and their receptors are widely expressed throughout the central nervous system, under both physiological and pathological conditions (2), and are involved to a variable extent in programmed cell death, apoptosis. For instance, a trophic, cytoprotective effect of IL-8 has been described on cultured hippocampal neurons (3) and on astrocytes (4) whereas the chemokine stromal-derived growth factor-1α has been shown to induce apoptosis in hNT neurons, a human cell line with neuronal characteristics (5), and cerebellar granule cells (6). We have recently found that cultured rat cerebellar granule neurons express functional IL-8RB/CXCR2 receptors and demonstrated that their stimulation by GROβ induces the activation of various signal transduction pathways, including the extracellular signal-regulated kinases, whose activation is involved in modulation of neurotransmitter release (7, 8). Considering that chemokines, by stimulating IL-8RB/CXCR2, can also activate the phosphatidylinositol 3-kinase/Akt signaling pathway (9), which plays an important inhibitory role in the development of apoptotic processes (10, 11), we decided to examine whether the IL-8RB/CXCR2 agonist GROβ would affect the survival of cultured cerebellar granules. Furthermore, because it is known that cerebellar granule neuron growth and development, both in vitro and in vivo, is greatly dependent on excitatory amino acids (12), the potential involvement of glutamate receptors (GluRs) on possible GROβ neuroprotective effects was also investigated.
Materials and Methods
Materials.
Rat GROβ was from PeproTech (Rocky Hill, NJ) and Chemicon; polyclonal Abs for GluR subunits were from Chemicon; 2-amino-5-phosphonopentanoic acid (AP-5), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), N-methyl-d-aspartate (NMDA), and GYKI 52466 were from Sigma-Aldrich; antisense oligonucleotides were from Eurogentech (Brussels); and nitrocellulose paper and enhanced chemiluminescence (ECL) were from Amersham Pharmacia.
Primary Cerebellar Granule Cultures.
Cerebellar granule cells were obtained from 8-day-old Wistar rats (13) and cultured in basal Eagle's medium containing 25 mM K+ and 10% heat-inactivated FCS. To prevent replication of non-neural cells, after 24 h, the cultures were supplemented with cytosine arabinoside (10 μM); these cultures contained >95% granule neurons (14).
Neuron Survival Assay.
Cells (≈5 × 105) were seeded on poly-L-lysine-coated plastic 24-well (15.5 mm) dishes. After 6 days of culture the cells were switched to serum-free basal Eagle's medium containing 5 mM K+ to induce apoptosis or kept in 25 mM K+ medium for control, as described (15). After 24 h, the cells were detached with a detergent-containing lysing solution and counted in a hemacytometer for viability, as described (16).
Hoechst Staining.
Cells (≈5 × 105) were seeded on 12-mm coverslips placed in 15.5-mm dishes. To visualize nuclear morphology, the cells were fixed for 15 min in 4% paraformaldehyde, permeabilyzed for 5 min with 0.2% Triton X-100 in 0.1 M Tris⋅HCl (pH 7.4) and incubated for 5 min with 1 μg/ml of the DNA dye Hoechst 33258. Stained nuclei were examined with a ×50 objective, and five different fields were counted for each coverslip. Each field contained about 150 cells. Condensed or fragmented nuclei were scored as apoptotic, whereas uniformly stained nuclei were considered as viable cells (10).
Cell Treatment with Antisense Oligonucleotides.
Phosphorothioate oligodeoxynucleotide (14 bases long) corresponding to the sequence common to the cDNAs of the four AMPA receptor subunits was synthesized in the antisense orientation. This antisense, here named αA (5′-ACACAGTAGCCYTC-3′; Y = A + G), is complementary to positions 1306–1319 of the AMPA type GluR1, 1327–1340 of GluR2, 1336–1349 of GluR3, and 1330–1343 of GluR4. In the “scrambled” antisense, here designated αS (5′-ATCACTCGCAAGYC-3′), the 14-mer αA was randomized and used as control. The sequence of αS, compared with sequences deposited in the GenBank/EMBL data banks, revealed no identity with other rodent sequences. Rat granule cells cultured for 3 days were treated with the antisense (10 μM) added fresh every 36 h (three additions in total). Six- or 7-day cultured cells were lysed for Western blot analysis, treated for survival assay, or used for electrophysiological recordings.
Western Blot Analysis.
Cerebellar granule cells were scraped off the plate in a buffer containing 10 mM Tris⋅HCl, 2 mM EGTA, 1 mM MgCl2 (pH 7.5), 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin, and centrifuged for 60 min at 100,000 rpm in an airfuge to separate membranes from the cytosol fraction. The membranes were then resuspended in buffer containing 0.5% Triton X-100 and 0.5% Tween 20 and sonicated for 5 s on ice with a probe sonicator. Equivalent amounts of membrane proteins were analyzed on 7.5% SDS polyacrylamide gel and electrophoretically transferred to nitrocellulose paper for 1 h at 4°C. Blots were incubated for 2 h with 3% BSA to block nonspecific binding sites and then incubated with affinity-purified rabbit antibodies directed against AMPA-type GluR subunits. The immunoreactivity was detected with a chemiluminescent substrate (enhanced chemiluminescence).
Electrophysiological Recordings in Cerebellar Granules.
Whole-cell patch-clamped cerebellar granule neurons were superfused with oxygenated saline (1.5–2.0 ml/min). The intracellular solution contained 140 mM CsCl, 2 mM MgCl2, 10 mM Hepes, 4 mM Na-ATP, 0.5 mM EGTA, or 2 mM BAPTA [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate], pH 7.3. Membrane currents were recorded and filtered (2 kHz) with an Axopatch 200A (Axon Instruments, Foster City, CA), stored, and analyzed by using pClamp software (Axon Instruments). Drugs were bath applied with a gravity-driven perfusion system with a delay of about 4 s. Neurons were held at −75 mV. For more details, see refs. 8 and 17.
Results and Discussion
GROβ and IL-8 Rescue Cerebellar Granule Neurons from Apoptotic Death Induced by Low K+.
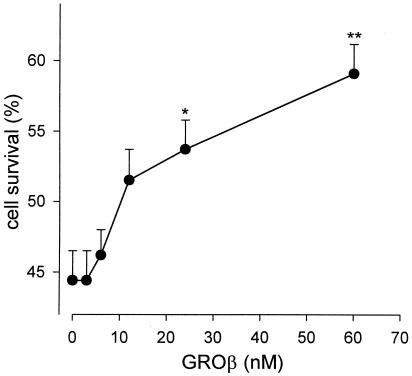
We investigated the effects of GROβ on the survival of rat granule cells grown for 6 days in high K+ medium and then transferred to low K+ medium. Six-day cultured granule neurons are dependent on membrane depolarization for survival (18), and upon exposure to a medium containing 5 mM K+ (low K+) the cells are induced to die by activation of apoptotic processes that cause about 50% of the cells to die within 24 h (15). Accordingly, we observed that 44.4 ± 2.1% of cultured granule cells survived in 5 mM K+ medium, as determined by counting the number of intact nuclei after cell lysis. Furthermore, we found that GROβ increased cell survival in a dose-dependent manner, as shown in Fig. 1. Specifically, in the presence of 60 nM GROβ, cell survival increased to 59.1 ± 2.1% after 24 h, with a net increase of 33%. Higher concentrations of GROβ did not increase further the percentage of surviving cells. The protective effect of GROβ was also evaluated by counting the number of cells containing condensed and fragmented chromatin after staining the neurons with Hoechst 33342. This different way of estimating cell survival gave qualitatively similar results (Table 1). Comparable protection from apoptotic death was also obtained with IL-8; because IL-8 is a human chemokine, we decided to perform further experiments with GROβ (a rat chemokine).
Figure 1.
Dose-dependence of GROβ-mediated neuronal survival. Granule cell neurons cultured for 6 days in 25 mM K+ medium were switched to 5 mM K+-containing medium and incubated in the presence of increasing concentrations of GROβ. After 24 h, the cells were lysed and nuclei counted as described in Materials and Methods. Data are expressed as number of surviving cells in 5 mM K+ medium vs 25 mM K+ (taken as 100%), and are the mean ± SEM of 6–15 independent experiments. Student's t test: **, P < 0.00001; *, P < 0.001.
Table 1.
GROβ reduces the number of apoptotic nuclei in cerebellar granule cells
| Medium | Apoptotic nuclei, % of total cells |
|---|---|
| K+ (25 mM) | 5.0 ± 0.3 |
| K+ (5 mM) | 29.3 ± 2.0 |
| K+ (5 mM) + GROβ | 16.0 ± 0.9 |
Six-day cultured cells were kept for a further 24 h in 5 mM K+-containing medium in the presence or in the absence of 60 nM GROβ, and treated with the fluorescent dye Hoechst 33258. Data indicate the number of apoptotic nuclei as percentage of total cells and represent the mean ± SEM of four independent experiments. Student's t test paired analysis of K+ 5 mM vs. K+ 5 mM + GROβ-treated cells: P < 0.0001.
The Neuroprotective Effect of GROβ Is Blocked by AMPA Receptor Antagonists.
Cerebellar granule neurons express both ionotropic (NMDA and AMPA/kainate) and metabotropic GluRs, and the expression levels for the different receptor subunits vary during in vitro cellular development and are dependent on the culture conditions (19, 20). It is known also that granule cell survival, both in vivo and in vitro, can be modulated by stimulating different GluRs, and that this GluR dependence is correlated with the age of the animal and of the culture (21–23). Therefore, we investigated whether the neuroprotective effect of GROβ is mediated by activation of ionotropic GluRs; and for that purpose we used specific inhibitors for NMDA (AP-5) and for AMPA/kainate (CNQX) receptors. The results illustrated in Fig. 2 show that the protective effect of the chemokine was completely lost after treating the cells with 10 μM CNQX, whereas it was unaltered by exposure to 10 μM AP-5. Moreover, a complete inhibition of the effect of GROβ was also obtained with a different inhibitor of AMPA receptors, namely 100 μM GYKI 52466 (data not shown). Exposing the cells to CNQX also completely abolished the antiapoptotic activity of IL-8 (data not shown). When the neurons were treated with 10 μM AMPA in 5 mM K+, it exerted an effect on cell survival that was comparable to that obtained with GROβ (respectively, 71 ± 8% and 68.4 ± 4% in the set of experiments shown in Fig. 3). The effect of AMPA was not additive with that of GROβ (AMPA + GROβ led to 77.7 ± 8% survival, Fig. 3), suggesting that both substances use a common mechanism to mediate neuroprotection. Because CNQX blocked the protective effect of GROβ on neuronal death, it was important to ascertain whether the CNQX used blocked the GluRs of the cultured granule cells. Electrophysiological recordings from whole-cell patch-clamped granule neurons confirmed that the concentrations of GluR inhibitors used were sufficient to block the effect of the corresponding agonist. As already reported (24), the effect of 100 μM NMDA on cell survival was more pronounced (90.6 ± 27%; Fig. 3) than that of AMPA, and it was blocked by 10 μM AP-5 (data not shown). The effect of AMPA on cell survival was also efficiently blocked by 10 μM CNQX or by 100 μM GYKI 52466 (data not shown). Furthermore, we performed the survival experiments in the presence of 10–50 μM cyclothiazide, a drug used frequently to inhibit AMPA receptor desensitization (25). We found that the effect of AMPA on granule cell survival was not enhanced upon cyclothiazide treatment (data not shown), suggesting that the duration of receptor activation is not important for the AMPA-induced neurotrophic effect, which is in agreement with data reported elsewhere (26).
Figure 2.
Effect of GluR inhibitors on granule cell survival. Cultured neurons were treated for 24 h with vehicle (C), 10 μM CNQX, or 10 μM AP-5, in the presence or in the absence of 60 nM GROβ, in 5 mM K+-containing medium, as described in the legend to Fig. 1. Data are expressed as in Fig. 1 and are the mean ± SEM of at least six independent experiments. For statistic analysis, each group of cells was compared with the corresponding control: C vs GROβ; CNQX vs CNQX + GROβ; AP-5 vs AP-5 + GROβ. Student's t test: *, P < 0.001.
Figure 3.
Effects of GROβ and GluR agonists on granule cell survival. Cultured neurons were treated for 24 h with vehicle (C), 60 nM GROβ, 10 μM AMPA, 100 μM NMDA, or 60 nM GROβ + 10 μM AMPA, in 5 mM K+-containing medium, as described in the legend to Fig. 1. Data expressed as in Fig. 1 are the mean ± SEM of at least six independent experiments. Student's t test: *, P < 0.001.
Effect of GluR Antisense on GROβ Neuroprotection.
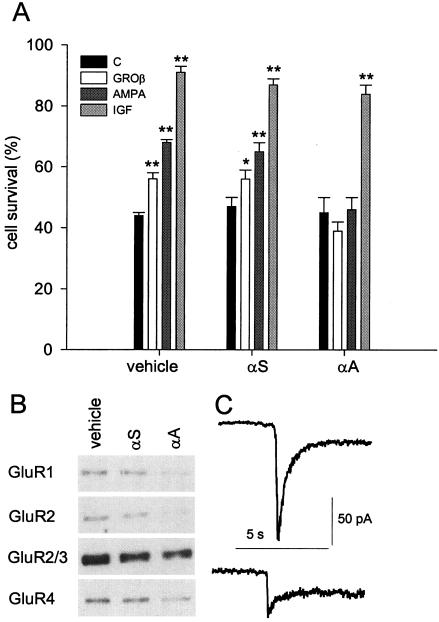
To inhibit AMPA receptor expression, cerebellar granule neurons were treated with 10 μM antisense oligonucleotide specific for a sequence in common with all four AMPA receptor subunits (αA) three times at 36-h intervals, starting at 3 days. Control cultures were treated similarly but with a scrambled antisense (αS) used as control or with vehicle. Neurons treated with either αA or αS were viable and, when switched to medium containing 5 mM K+, the number of cells that survived was similar (45 ± 5% for αA and 47 ± 3% for αS) to untreated cultures (44 ± 1%, Fig. 4A). Nevertheless, the neurotrophic effect of both GROβ and AMPA was not inducible in cultures treated with αA, although in cultures treated with αS it was still the same as for cultures not exposed to an antisense (Fig. 4A). This indicates that the effect of αA was specific and was not caused by antisense toxicity. Treatment with αA also efficiently blocked IL-8-mediated cell survival (data not shown). Furthermore, insulin-like growth factor still stimulated the survival of αA-treated cells as efficiently as in vehicle-treated cells (Fig. 4A), suggesting that the machinery of the apoptotic/antiapoptotic process was intact after antisense treatment. To assess AMPA receptor subunit expression, Western blot analysis with subunit-specific Abs was performed on antisense-treated or control cultures. As expected, treating the cultured granule cells with αA reduced the expression of all of the four AMPA receptor subunits, as in the representative experiment illustrated in Fig. 4B. This figure also shows a minor reduction in subunit expression in cultures treated with αS, possibly reflecting some nonspecific effects of the oligonucleotide treatment. The effect of αA treatment on AMPA receptor expression was evaluated also by electrophysiological recordings of AMPA-mediated currents in granule cells. Fig. 4C shows that in αA-treated cells AMPA (30 μM) induced a considerably smaller current, indicating that the antisense oligonucleotide treatment efficiently diminished the expression of AMPA receptors.
Figure 4.
Effects of antisense treatment on granule cells. (A) Cultured granule neurons were incubated with vehicle, αS, or αA as described in Material and Methods, and then were not treated (C), or treated with 60 nM GROβ, 10 μM AMPA, or 25 ng/ml insulin-like growth factor (IGF) for 24 h. Data expressed as in Fig. 1. Student's t test: ∗, P < 0.05; ∗∗, P < 0.001. (B) Western blot analysis of the expression of GluR subunits in cultures treated with vehicle, αS or αA. Band intensity quantification for the experiment shown (representative of four different determinations) revealed the following values (expressed as percentage of untreated cultures): GluR1: αS, 79%; αA, 9.5%; GluR2: αS, 63%; αA, 3%; GluR2/3: αS, 73%; αA, 56%; GluR4: αS, 83%; αA 30%. (C) Average currents from AMPA-stimulated nerve cells (30 μM; n = 10) untreated (Upper) or treated with αA (Lower) were, respectively 158 ± 39 pA (SEM, Student's t test, P < 0.05) and 60 ± 14 pA (SEM, Student's t test P < 0.05). Note larger reduction of AMPA currents in C compared with the GluR subunits expression in αA-treated neurons in B, which is attributed to the selection of most viable (likely antisense-underloaded) cells in C.
GROβ-Mediated Neuroprotection Does Not Depend on the Gating of AMPA Receptors.
We have investigated the possibility that the neuroprotective effect of GROβ depends on postsynaptic currents generated by GluRs activated by synaptically released glutamate. Our hypothesis was that activating CXCR2, which is known to raise intracellular Ca2+ (7), could lead to release of glutamate. Previously we have shown that GROβ modulates the spontaneous release from GABAergic nerve terminals making contacts with Purkinje neurons in cerebellar slices (17, 27). To address this issue, we performed both release experiments, prelabeling the intracellular pool of glutamate, and whole-cell patch-clamp recordings from cultured granule neurons acutely exposed to GROβ. We failed to detect either a significant increase of radioactive neurotransmitter in the medium bathing GROβ-stimulated cells (data not shown) or any obvious whole-cell current activated by GROβ cells (data not shown). This result suggests that mechanisms different from increased neurotransmitter release may be involved. In addition, whole cell currents activated by AMPA were not influenced by brief (5-min) or long-term (24-h) treatment with GROβ, indicating that GROβ did not influence the gating properties or the expression levels of AMPA receptors (data not shown).
In conclusion, our results show that the chemokine GROβ has an antiapoptotic activity on cultured cerebellar granule cells and reduces the number of cells that die upon K+ deprivation. This neuroprotection is not a direct effect of the chemokine, but is mediated through AMPA receptors being inhibited by CNQX, which specifically blocks non-NMDA receptors, and by an antisense specific for all of the subunits of AMPA receptors. What is still not entirely clear is whether this effect is mediated by synaptically released glutamate or whether GROβ somehow directly affects AMPA receptor function, perhaps through AMPA receptor-interacting proteins (28), or through the regulators of G protein signaling that contain, at their N terminus, a PDZ domain which has been shown to interact both with CXCR2 and GluR1 (29). We favor this last hypothesis, because we could not detect an increase in glutamate release after GROβ treatment. The effects of AMPA receptor activation have been classically related to Na+ and Ca2+ influx through the receptor channel. However, two recent reports describe a direct association/regulation of neuronal AMPA receptor subunits with the tyrosine kinase lyn (30) and with the α subunit of the inhibitory guanine-nucleotide-binding protein Gαi1 (31), indicating that AMPA receptor stimulation may include a complex cross-talk between different intracellular signaling pathways. To our knowledge, this is the first report that describes an antiapoptotic effect of a chemokine as being mediated through a neurotransmitter receptor. Our data lend further support to the increasing evidence of the importance of chemokines in some physiopathological events of the central nervous system and demonstrate that molecules (the chemokines) which classically belong to a foreign system compartment (the immune system) exert some of their effects on neurons by using the communication system of the central nervous system (neurotransmitter receptors).
Acknowledgments
We thank Drs. Giulio Levi and Angelo Spinedi for critical reading of the manuscript. This work was supported partly by grants from the Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica (to F.E. and A.S.), and from Telethon (no. E0912 to F.E.).
Abbreviations
- GROβ
growth-related gene product β
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- GluRs
glutamate receptors
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- AP-5
2-amino-5-phosphonopentanoic acid
- IL 8-RB/CXCR2
interleukin 8 receptor type B
- NMDA
N-methyl-d-aspartate
- αA
AMPA receptor subunit
- αS
scrambled antisense subunit
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090105997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090105997
References
- 1.Baggiolini M. Nature (London) 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 2.Hesselgesser J, Horuk R. J NeuroVirol. 1999;5:13–26. doi: 10.3109/13550289909029741. [DOI] [PubMed] [Google Scholar]
- 3.Araujo D M, Cotman C W. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- 4.Saas P, Boucraut J, Quiquerez A L, Schnuriger V, Perrin G, Desplat-Jego S, Bernard D, Walker P R, Dietrich P Y. J Immunol. 1999;162:2326–2333. [PubMed] [Google Scholar]
- 5.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson D L, Horuk R. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 6.Zheng J, Thylin M R, Ghorpade A, Xiong H, Persidsky Y, Cotter R L, Niemann D, Che M, Zeng Y-C, Gelbard H A, et al. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 7.Limatola C, Mileo A M, Giovannelli A, Vacca F, Ciotti M T, Mercanti D, Santoni A, Eusebi F. J Biol Chem. 1999;274:36537–36543. doi: 10.1074/jbc.274.51.36537. [DOI] [PubMed] [Google Scholar]
- 8.Ragozzino D, Giovannelli A, Mileo A M, Limatola C, Santoni A, Eusebi F. NeuroReport. 1998;9:3601–3606. doi: 10.1097/00001756-199811160-00011. [DOI] [PubMed] [Google Scholar]
- 9.Tilton B, Andjelkovic M, Didichenko A, Hemmings B A, Thelen M. J Biol Chem. 1997;272:28096–28101. doi: 10.1074/jbc.272.44.28096. [DOI] [PubMed] [Google Scholar]
- 10.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 11.Crowder R J, Freeman R S. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balazs R, Hack N, Jorgensen O S. In: Drug Research Related to Neuroactive Amino Acids. Schousboe A, Diemer N H, Kofold H, editors. Copenhagen: Munksgaard; 1992. pp. 397–410. [Google Scholar]
- 13.Gallo V, Giovanini C, Levi G. J Neurochem. 1990;54:1619–1625. doi: 10.1111/j.1471-4159.1990.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 14.Levi G, Wilkin G P, Ciotti M T, Johnstone S. Brain Res. 1983;312:227–241. doi: 10.1016/0165-3806(83)90139-6. [DOI] [PubMed] [Google Scholar]
- 15.D'Mello S R, Galli C, Ciotti T, Calissano P. Proc Natl Acad Sci USA. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volontè C, Ciotti M T, Battistini L. Cytometry. 1994;17:274–276. doi: 10.1002/cyto.990170311. [DOI] [PubMed] [Google Scholar]
- 17.Giovannelli A, Limatola C, Ragozzino D, Mileo A M, Ruggieri A, Ciotti M T, Mercanti D, Santoni A, Eusebi F. J Neuroimmunol. 1998;92:122–132. doi: 10.1016/s0165-5728(98)00192-1. [DOI] [PubMed] [Google Scholar]
- 18.Kingsbury A E, Gallo V, Woodhams P L, Balazs R. Brain Res. 1985;349:17–25. doi: 10.1016/0165-3806(85)90128-2. [DOI] [PubMed] [Google Scholar]
- 19.Aronica E, Dell'Albani P, Condorelli D F, Nicoletti F, Hack N, Balazs R. Mol Pharmacol. 1994;44:981–989. [PubMed] [Google Scholar]
- 20.Condorelli D F, Dell'Albani P, Aronica E, Genazzani A A, Casabona G, Corsaro M, Balazs R, Nicoletti F. J Neurochem. 1993;61:2133–2139. doi: 10.1111/j.1471-4159.1993.tb07451.x. [DOI] [PubMed] [Google Scholar]
- 21.Balazs R, Jorgenson O S, Hack N. Neuroscience. 1988;27:437–451. doi: 10.1016/0306-4522(88)90279-5. [DOI] [PubMed] [Google Scholar]
- 22.Balazs R, Hack N, Jorgenson O S. Neuroscience. 1990;37:251–258. doi: 10.1016/0306-4522(90)90211-l. [DOI] [PubMed] [Google Scholar]
- 23.Copani A, Bruno V M G, Barresi V, Battaglia G, Condorelli D F, Nicoletti F. J Neurochem. 1995;64:101–108. doi: 10.1046/j.1471-4159.1995.64010101.x. [DOI] [PubMed] [Google Scholar]
- 24.Hack N, Hidaka H, Wakefield M J, Balazs R. J Neurosci. 1993;57:9–20. doi: 10.1016/0306-4522(93)90108-r. [DOI] [PubMed] [Google Scholar]
- 25.Yamada K A, Tang C M. J Neurosci. 1993;13:3904–3915. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hack N, Balazs R. Neurochem Int. 1994;25:235–241. doi: 10.1016/0197-0186(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 27.Ragozzino D, Giovannelli A, Mileo A M, Limatola C, Santoni A, Eusebi F. NeuroReport. 1998;9:3601–3606. doi: 10.1097/00001756-199811160-00011. [DOI] [PubMed] [Google Scholar]
- 28.Xia J, Zhang X, Staudinger J, Huganir R L. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 29.Snow B E, Hall R A, Krumins A M, Brothers G M, Bouchard D, Brothers C A, Chung S, Mangion J, Gilman A G, Lefkowitz R J, Siderovski D P. J Biol Chem. 1998;273:17749–17755. doi: 10.1074/jbc.273.28.17749. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi T, Umemori H, Mishina M, Yamamoto T. Nature (London) 1999;397:72–76. doi: 10.1038/16269. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Small D, L, Stanirimovic D B, Morley P, Durkin J P. Nature (London) 1997;389:502–504. doi: 10.1038/39062. [DOI] [PubMed] [Google Scholar]