Abstract
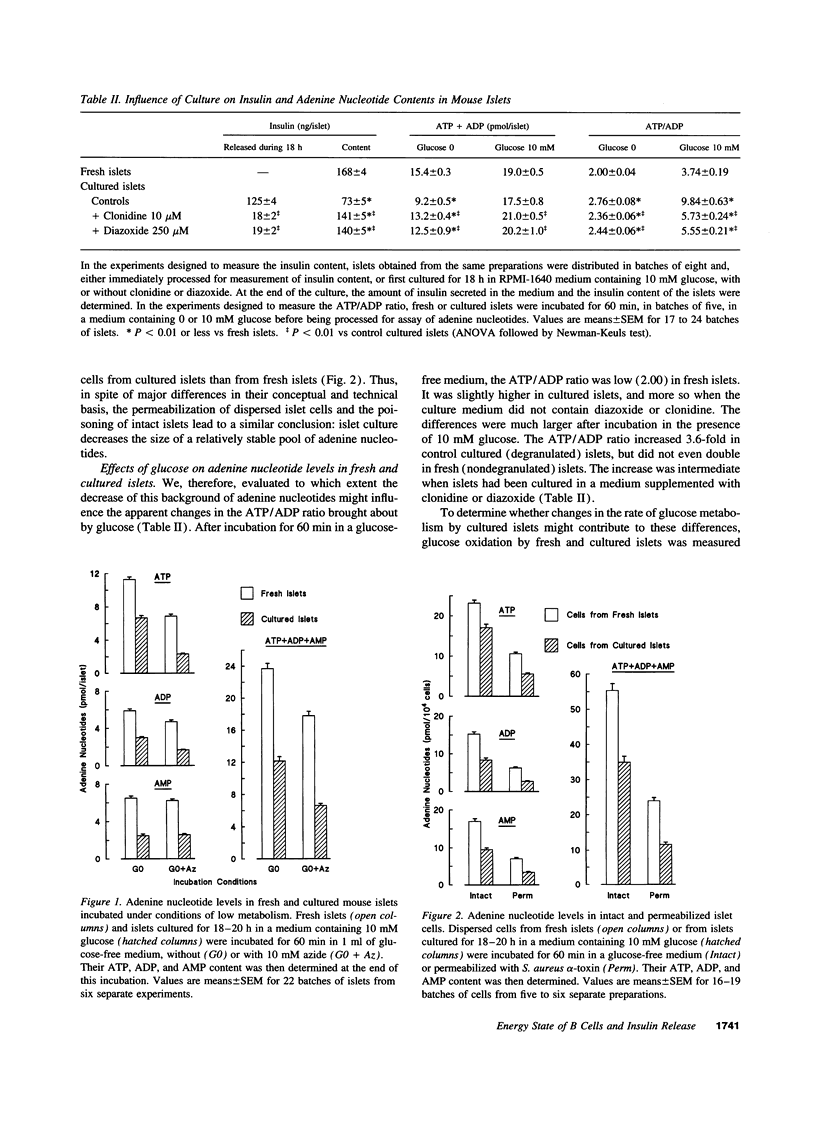
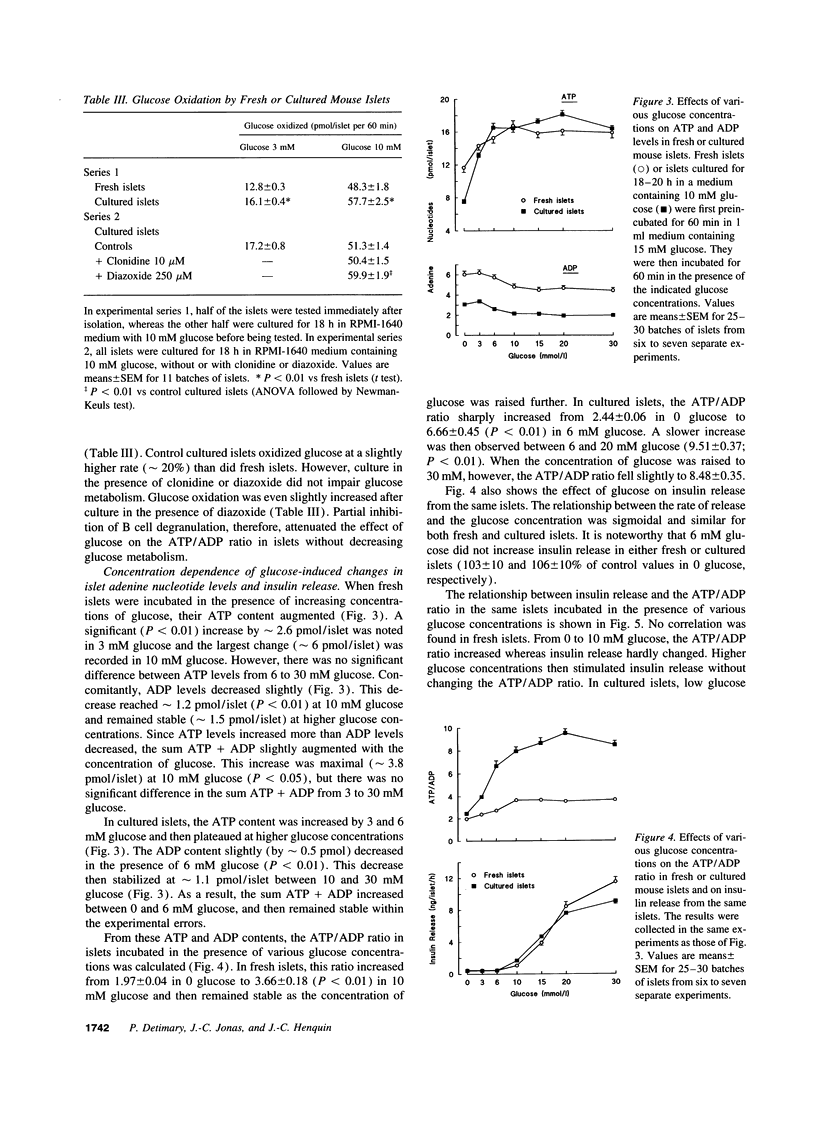
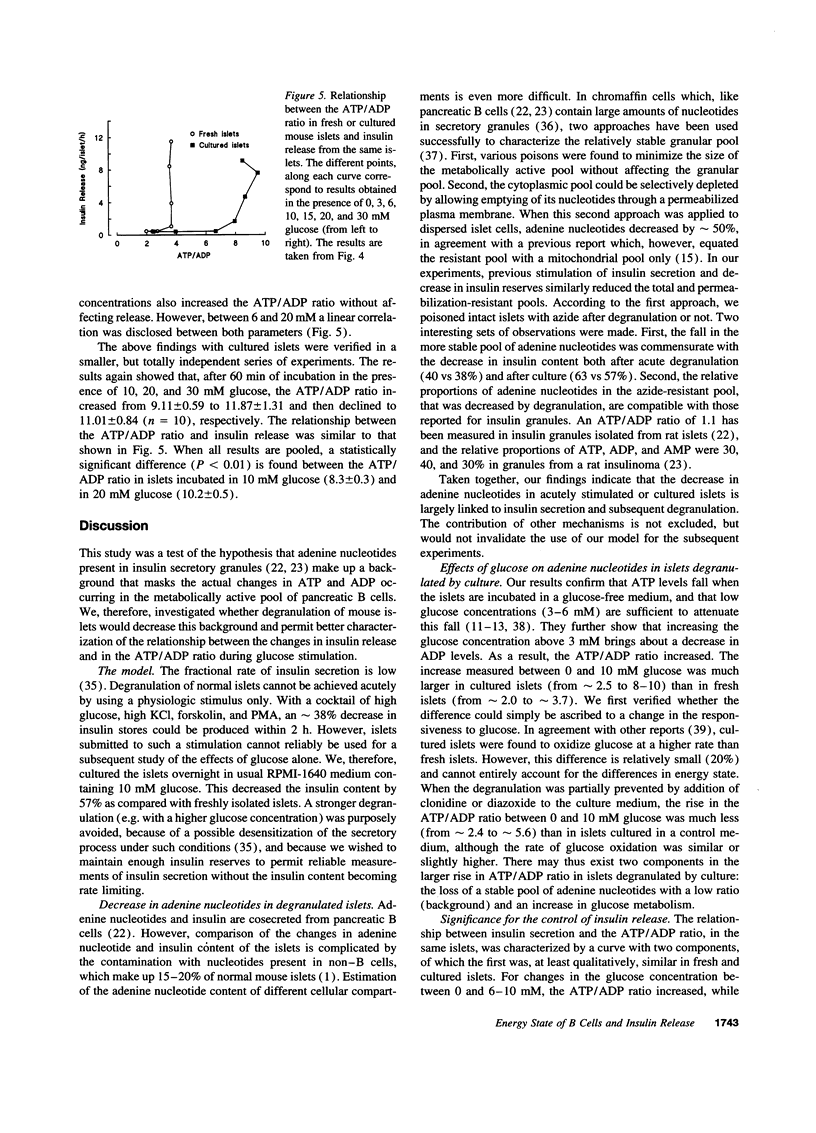
Whether adenine nucleotides in pancreatic B cells serve as second messengers during glucose stimulation of insulin secretion remains disputed. Our hypothesis was that the actual changes in ATP and ADP are obscured by the large pool of adenine nucleotides (ATP/ADP ratio close to 1) in insulin granules. Therefore, mouse islets were degranulated acutely with a cocktail of glucose, KCl, forskolin, and phorbol ester or during overnight culture in RPMI-1640 medium containing 10 mM glucose. When these islets were then incubated in 0 glucose + azide (to minimize cytoplasmic and mitochondrial adenine nucleotides), their content in ATP + ADP + AMP was decreased in proportion to the decrease in insulin stores. After incubation in 10 mM glucose (no azide), the ATP/ADP ratio increased from 2.4 to > 8 in cultured islets, and only from 2 to < 4 in fresh islets. These differences were not explained by changes in glucose oxidation. The glucose dependency (0-30 mM) of the changes in insulin secretion and in the ATP/ADP ratio were then compared in the same islets. In nondegranulated, fresh islets, the ATP/ADP ratio increased between 0 and 10 mM glucose and then stabilized although insulin release kept increasing. In degranulated islets, the ATP/ADP ratio also increased between 0 and 10 mM glucose, but a further increase still occurred between 10 and 20 mM glucose, in parallel with the stimulation of insulin release. In conclusion, decreasing the granular pool of ATP and ADP unmasks large changes in the ATP/ADP ratio and a glucose dependency which persists within the range of stimulatory concentrations. The ATP/ADP ratio might thus serve as a coupling factor between glucose metabolism and insulin release.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Mach W., Föhr K. J., Gratzl M. Poration by alpha-toxin and streptolysin O: an approach to analyze intracellular processes. Methods Cell Biol. 1989;31:63–90. doi: 10.1016/s0091-679x(08)61602-7. [DOI] [PubMed] [Google Scholar]
- Andersson A., Hellerström C. Metabolic characteristics of isolated pancreatic islets in tissue culture. Diabetes. 1972;21(2 Suppl):546–554. doi: 10.2337/diab.21.2.s546. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Randle P. J. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973 Feb;132(2):223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran J. J., Korner M., Caughey B., Kirshner N. Metabolic pools of ATP in cultured bovine adrenal medullary chromaffin cells. J Neurochem. 1986 Sep;47(3):945–952. doi: 10.1111/j.1471-4159.1986.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Detimary P., Gilon P., Nenquin M., Henquin J. C. Two sites of glucose control of insulin release with distinct dependence on the energy state in pancreatic B-cells. Biochem J. 1994 Feb 1;297(Pt 3):455–461. doi: 10.1042/bj2970455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Potassium selective ion channels in insulin-secreting cells: physiology, pharmacology and their role in stimulus-secretion coupling. Biochim Biophys Acta. 1991 Mar 7;1071(1):67–82. doi: 10.1016/0304-4157(91)90012-l. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Bryła J., Michalik M., Meglasson M. D., Nelson D. Energy metabolism in islets of Langerhans. Biochim Biophys Acta. 1992 Aug 7;1101(3):273–295. doi: 10.1016/0005-2728(92)90084-f. [DOI] [PubMed] [Google Scholar]
- Gembal M., Detimary P., Gilon P., Gao Z. Y., Henquin J. C. Mechanisms by which glucose can control insulin release independently from its action on adenosine triphosphate-sensitive K+ channels in mouse B cells. J Clin Invest. 1993 Mar;91(3):871–880. doi: 10.1172/JCI116308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Ronner P., Cheong E., Khalid P., Matschinsky F. M. The role of ATP and free ADP in metabolic coupling during fuel-stimulated insulin release from islet beta-cells in the isolated perfused rat pancreas. J Biol Chem. 1991 Dec 5;266(34):22887–22892. [PubMed] [Google Scholar]
- Grodsky G. M., Bolaffi J. L. Desensitization of the insulin-secreting beta cell. J Cell Biochem. 1992 Jan;48(1):3–11. doi: 10.1002/jcb.240480103. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Danielsson A. Adenosine triphosphate levels of mammalian pancreatic B cells after stimulation with glucose and hypoglycemic sulfonylureas. Diabetes. 1969 Aug;18(8):509–516. doi: 10.2337/diab.18.8.509. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. ATP-sensitive K+ channels may control glucose-induced electrical activity in pancreatic B-cells. Biochem Biophys Res Commun. 1988 Oct 31;156(2):769–775. doi: 10.1016/s0006-291x(88)80910-0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. D-glucose inhibits potassium efflux from pancreatic islet cells. Nature. 1978 Jan 19;271(5642):271–273. doi: 10.1038/271271a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Lambert A. E. Bicarbonate modulation of glucose-9nduced biphasic insulin release by rat islets. Am J Physiol. 1976 Sep;231(3):713–721. doi: 10.1152/ajplegacy.1976.231.3.713. [DOI] [PubMed] [Google Scholar]
- Hopkins W. F., Fatherazi S., Peter-Riesch B., Corkey B. E., Cook D. L. Two sites for adenine-nucleotide regulation of ATP-sensitive potassium channels in mouse pancreatic beta-cells and HIT cells. J Membr Biol. 1992 Sep;129(3):287–295. doi: 10.1007/BF00232910. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983 Feb 15;210(2):297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas J. C., Li G., Palmer M., Weller U., Wollheim C. B. Dynamics of Ca2+ and guanosine 5'-[gamma-thio]triphosphate action on insulin secretion from alpha-toxin-permeabilized HIT-T15 cells. Biochem J. 1994 Jul 15;301(Pt 2):523–529. doi: 10.1042/bj3010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Leitner J. W., Sussman K. E., Vatter A. E., Schneider F. H. Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology. 1975 Mar;96(3):662–677. doi: 10.1210/endo-96-3-662. [DOI] [PubMed] [Google Scholar]
- Longo E. A., Tornheim K., Deeney J. T., Varnum B. A., Tillotson D., Prentki M., Corkey B. E. Oscillations in cytosolic free Ca2+, oxygen consumption, and insulin secretion in glucose-stimulated rat pancreatic islets. J Biol Chem. 1991 May 15;266(14):9314–9319. [PubMed] [Google Scholar]
- MacDonald M. J. Elusive proximal signals of beta-cells for insulin secretion. Diabetes. 1990 Dec;39(12):1461–1466. doi: 10.2337/diab.39.12.1461. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Hutton J. C., Kawazu S., Herchuelz A., Valverde I., Sener A. The stimulus-secretion coupling of glucose-induced insulin release. XXXV. The links between metabolic and cationic events. Diabetologia. 1979 May;16(5):331–341. doi: 10.1007/BF01223623. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A. Glucose-induced changes in cytosolic ATP content in pancreatic islets. Biochim Biophys Acta. 1987 Feb 18;927(2):190–195. doi: 10.1016/0167-4889(87)90134-0. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Misler S., Barnett D. W., Gillis K. D., Pressel D. M. Electrophysiology of stimulus-secretion coupling in human beta-cells. Diabetes. 1992 Oct;41(10):1221–1228. doi: 10.2337/diab.41.10.1221. [DOI] [PubMed] [Google Scholar]
- Niki I., Ashcroft F. M., Ashcroft S. J. The dependence on intracellular ATP concentration of ATP-sensitive K-channels and of Na,K-ATPase in intact HIT-T15 beta-cells. FEBS Lett. 1989 Nov 6;257(2):361–364. doi: 10.1016/0014-5793(89)81572-8. [DOI] [PubMed] [Google Scholar]
- Ohta M., Nelson D., Nelson J., Meglasson M. D., Erecińska M. Oxygen and temperature dependence of stimulated insulin secretion in isolated rat islets of Langerhans. J Biol Chem. 1990 Oct 15;265(29):17525–17532. [PubMed] [Google Scholar]
- Palmer M., Jursch R., Weller U., Valeva A., Hilgert K., Kehoe M., Bhakdi S. Staphylococcus aureus alpha-toxin. Production of functionally intact, site-specifically modifiable protein by introduction of cysteine at positions 69, 130, and 186. J Biol Chem. 1993 Jun 5;268(16):11959–11962. [PubMed] [Google Scholar]
- Rajan A. S., Aguilar-Bryan L., Nelson D. A., Yaney G. C., Hsu W. H., Kunze D. L., Boyd A. E., 3rd Ion channels and insulin secretion. Diabetes Care. 1990 Mar;13(3):340–363. doi: 10.2337/diacare.13.3.340. [DOI] [PubMed] [Google Scholar]
- Scott D. A., Fisher A. M. THE INSULIN AND THE ZINC CONTENT OF NORMAL AND DIABETIC PANCREAS. J Clin Invest. 1938 Nov;17(6):725–728. doi: 10.1172/JCI101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A., Rorsman P., Ashcroft F. M. Modulation of dihydropyridine-sensitive Ca2+ channels by glucose metabolism in mouse pancreatic beta-cells. Nature. 1989 Nov 30;342(6249):550–553. doi: 10.1038/342550a0. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Trus M., Warner H., Matschinsky F. Effects of glucose on insulin release and on intermediary metabolism of isolated perifused pancreatic islets from fed and fasted rats. Diabetes. 1980 Jan;29(1):1–14. doi: 10.2337/diab.29.1.1. [DOI] [PubMed] [Google Scholar]
- Warnotte C., Gilon P., Nenquin M., Henquin J. C. Mechanisms of the stimulation of insulin release by saturated fatty acids. A study of palmitate effects in mouse beta-cells. Diabetes. 1994 May;43(5):703–711. doi: 10.2337/diab.43.5.703. [DOI] [PubMed] [Google Scholar]
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]
- Zhou X. J., Fadda G. Z., Perna A. F., Massry S. G. Phosphate depletion impairs insulin secretion by pancreatic islets. Kidney Int. 1991 Jan;39(1):120–128. doi: 10.1038/ki.1991.15. [DOI] [PubMed] [Google Scholar]


