Abstract
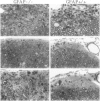
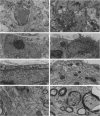
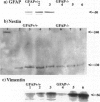
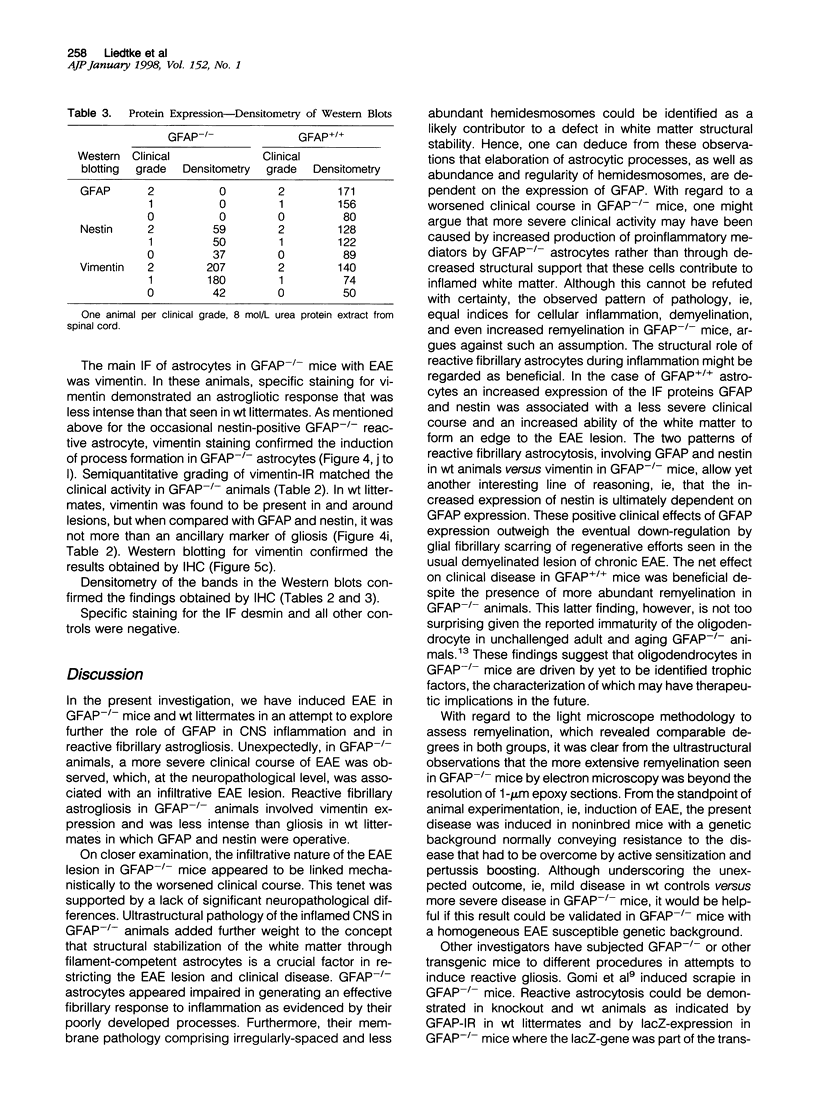
Insights into the role of the astrocyte intermediate filament protein, glial fibrillary acidic protein (GFAP), have only recently emerged with reports on subtle abnormalities in GFAP-deficient mice, including the documentation of defective long-term maintenance of central nervous system myelination. Here, we extend these observations by examining the astroglial response in GFAP-/- mice with autoimmune encephalomyelitis (EAE), a model for multiple sclerosis. Clinically, the monophasic disease was more severe in GFAP-/- mice than in wild-type littermates despite increased remyelination in the former. More in keeping with the clinical course was the observation of an infiltrative EAE lesion in GFAP-/- mice. GFAP-/- astrocytes had a reduced cytoarchitectural stability as evidenced by less abundant and irregularly spaced hemidesmosomes. The blunt GFAP-/- astrocyte processes possessed intermediate filaments consisting mainly of vimentin, though to a lesser degree than in the wild-type. In contrast, in wild-type littermates, GFAP was most abundant and nestin occurred at lower levels. Taken together, the present study introduces the novel concepts that GFAP plays an important role in the control of clinical disease associated with formation of a clearly defined edge to the EAE lesion and that GFAP is operative in the regulation of the intermediate filament components in reactive fibrillary astrogliosis.
Full text
PDF

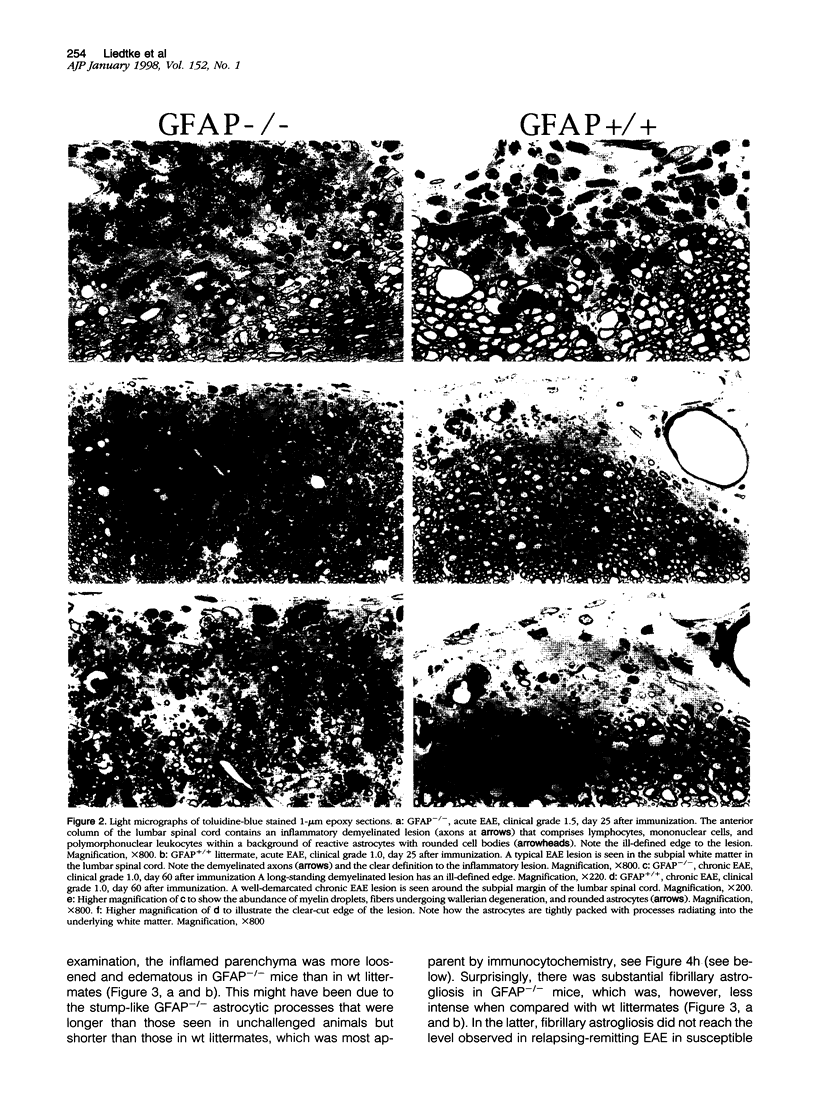
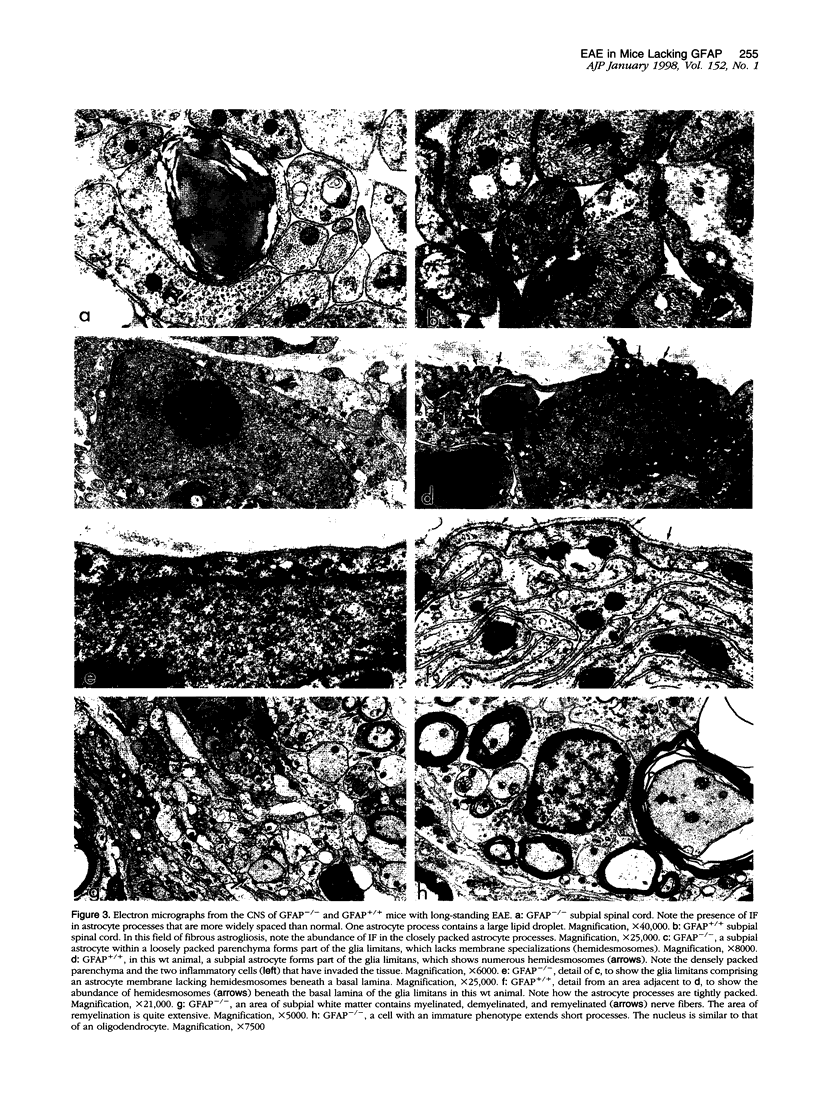
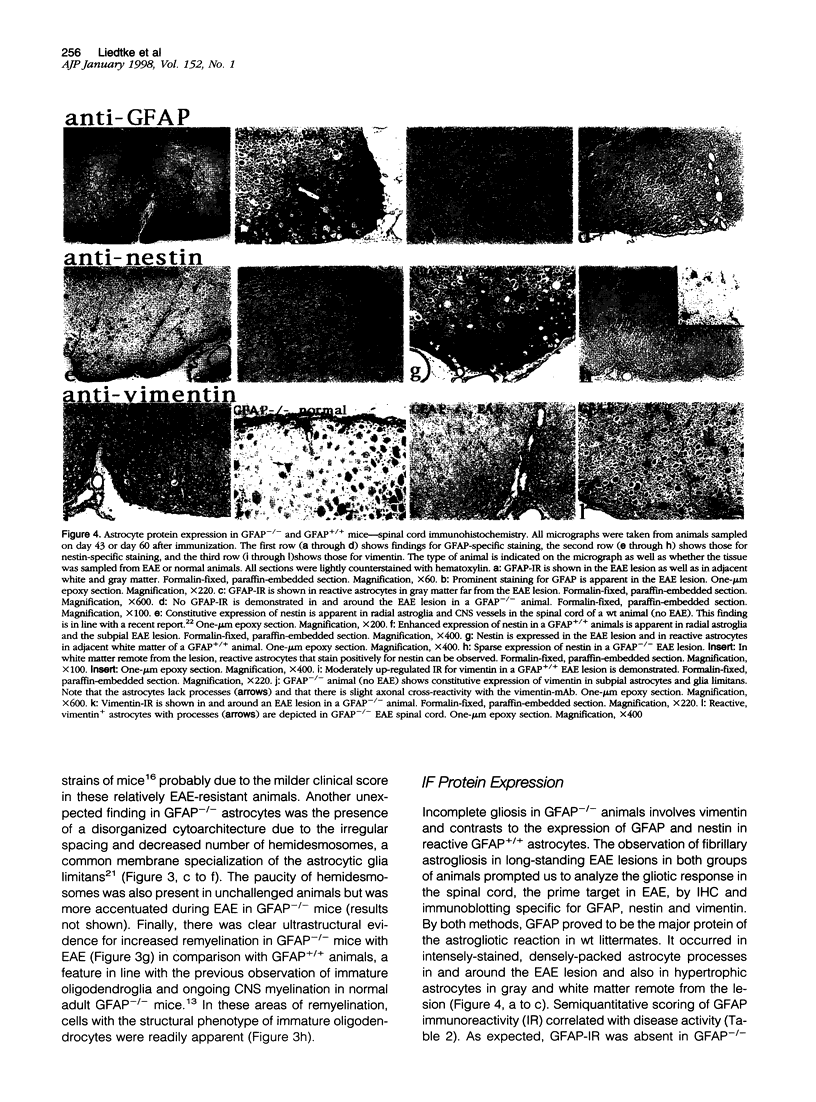
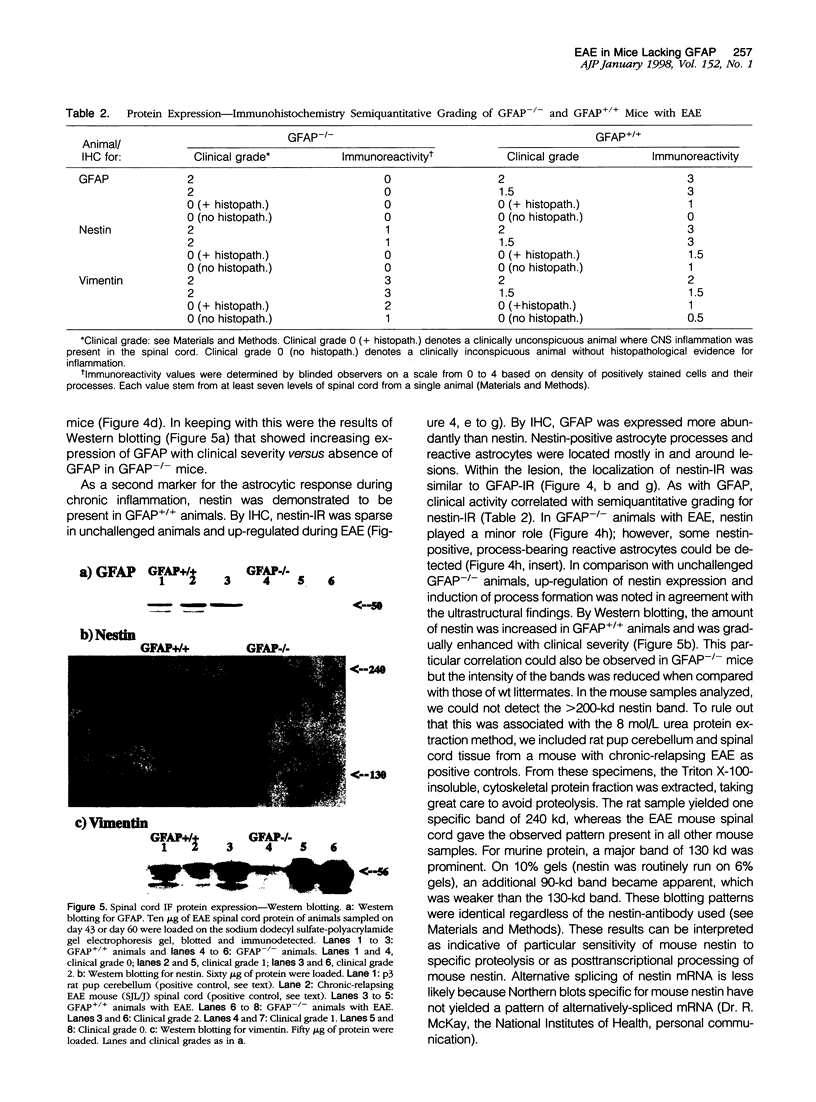

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eng L. F., Ghirnikar R. S. GFAP and astrogliosis. Brain Pathol. 1994 Jul;4(3):229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Frisén J., Johansson C. B., Török C., Risling M., Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995 Oct;131(2):453–464. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Keith R. Porter Lecture, 1996. Of mice and men: genetic disorders of the cytoskeleton. Mol Biol Cell. 1997 Feb;8(2):189–203. doi: 10.1091/mbc.8.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Galou M., Colucci-Guyon E., Ensergueix D., Ridet J. L., Gimenez y Ribotta M., Privat A., Babinet C., Dupouey P. Disrupted glial fibrillary acidic protein network in astrocytes from vimentin knockout mice. J Cell Biol. 1996 May;133(4):853–863. doi: 10.1083/jcb.133.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi H., Yokoyama T., Fujimoto K., Ikeda T., Katoh A., Itoh T., Itohara S. Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron. 1995 Jan;14(1):29–41. doi: 10.1016/0896-6273(95)90238-4. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J Neurosci. 1983 Nov;3(11):2206–2218. doi: 10.1523/JNEUROSCI.03-11-02206.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen K. R., Morgan L., Stewart H. J., Mirsky R. Three markers of adult non-myelin-forming Schwann cells, 217c(Ran-1), A5E3 and GFAP: development and regulation by neuron-Schwann cell interactions. Development. 1990 May;109(1):91–103. doi: 10.1242/dev.109.1.91. [DOI] [PubMed] [Google Scholar]
- Lendahl U., Zimmerman L. B., McKay R. D. CNS stem cells express a new class of intermediate filament protein. Cell. 1990 Feb 23;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Liedtke W., Edelmann W., Bieri P. L., Chiu F. C., Cowan N. J., Kucherlapati R., Raine C. S. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996 Oct;17(4):607–615. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- Martin R., McFarland H. F., McFarlin D. E. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- McCall M. A., Gregg R. G., Behringer R. R., Brenner M., Delaney C. L., Galbreath E. J., Zhang C. L., Pearce R. A., Chiu S. Y., Messing A. Targeted deletion in astrocyte intermediate filament (Gfap) alters neuronal physiology. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6361–6366. doi: 10.1073/pnas.93.13.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973 Oct;21(4):749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Pekny M., Levéen P., Pekna M., Eliasson C., Berthold C. H., Westermark B., Betsholtz C. Mice lacking glial fibrillary acidic protein display astrocytes devoid of intermediate filaments but develop and reproduce normally. EMBO J. 1995 Apr 18;14(8):1590–1598. doi: 10.1002/j.1460-2075.1995.tb07147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K., Gomi H., Chen L., Bao S., Kim J. J., Wakatsuki H., Fujisaki T., Fujimoto K., Katoh A., Ikeda T. Deficient cerebellar long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron. 1996 Mar;16(3):587–599. doi: 10.1016/s0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Skundric D. S., Huston K., Shaw M., Tse H. Y., Raine C. S. Experimental allergic encephalomyelitis. T cell trafficking to the central nervous system in a resistant Thy-1 congenic mouse strain. Lab Invest. 1994 Nov;71(5):671–679. [PubMed] [Google Scholar]
- Steinert P. M., Steven A. C., Roop D. R. The molecular biology of intermediate filaments. Cell. 1985 Sep;42(2):411–420. doi: 10.1016/0092-8674(85)90098-4. [DOI] [PubMed] [Google Scholar]
- Tohyama T., Lee V. M., Rorke L. B., Marvin M., McKay R. D., Trojanowski J. Q. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992 Mar;66(3):303–313. [PubMed] [Google Scholar]