Abstract
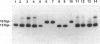
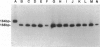
A 30-bp deletion in the Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) gene has been reported in nasopharyngeal carcinoma and EBV-associated malignant lymphomas. Information on this deletion in EBV-associated gastric carcinoma (EBVaGC) is limited. The association of gastric carcinoma (GC) with EBV was examined by EBV-encoded RNA (EBER) in situ hybridization in 510 patients from Japan and 80 patients from Brazil. We studied the prevalence of 30-bp LMP1 gene deletion in EBVaGC in Japan (29 cases) and Brazil (four cases) in comparison with the corresponding EBER1-positive metastatic lesions in lymph nodes (10 cases) and EBV-infected reactive lymphocytes from dissected nonmetastatic lymph nodes (22 cases), microdissected non-neoplastic gastric mucosa of EBVaGC (five cases), and EBV-nonassociated GC (25 cases). We studied the status of the LMP1 gene by Southern blot hybridization of polymerase chain reaction products obtained after amplification with primers flanking the site of the deletion. We also performed EBV typing and LMP1 protein immunohistochemistry. EBV DNA was amplified by polymerase chain reaction in 30 of 33 EBVaGC cases, 8 of 10 metastatic carcinomas, 14 non-neoplastic tissues from 27 EBVaGC cases, and 12 of 25 non-EBV-associated GC cases with EBER1-positive lymphocytes. The 30-bp LMP1 gene deletion was observed in 23 of 26 (88.5%) cases of EBVaGC from Japan and two of four (50%) cases of Brazilian EBVaGC as compared with EBER1-positive reactive lymphocytes from 11 of 14 (78.6%) EBVaGC cases and 9 of 12 (75%) cases of non-EBV-associated GC. The variant type (the 30-bp deletion variant or nondeleted wild type) of LMP1 gene was the same among reactive lymphocytes, primary and secondary lesions of EBVaGC in all cases for which all three tissue types were studied (six of six). There was no correlation between the presence of the 30-bp deletion with depth of cancer invasion or presence of metastasis. Type A was detected in all available EBV-positive cases. The similar high incidence of 30-bp deletion in LMP1 gene in both carcinoma cells and reactive lymphocytes in EBVaGC cases suggests that this deletion may not be relevant to the pathogenesis of EBVaGC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos I., Hummel M. Epstein-Barr virus in tumours. Histopathology. 1996 Oct;29(4):297–315. doi: 10.1111/j.1365-2559.1996.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Burke A. P., Yen T. S., Shekitka K. M., Sobin L. H. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990 May;3(3):377–380. [PubMed] [Google Scholar]
- Chang K. L., Chen Y. Y., Shibata D., Weiss L. M. Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn Mol Pathol. 1992 Dec;1(4):246–255. [PubMed] [Google Scholar]
- Chang Y. S., Su I. J., Chung P. J., Shu C. H., Ng C. K., Wu S. J., Liu S. T. Detection of an Epstein-Barr-virus variant in T-cell-lymphoma tissues identical to the distinct strain observed in nasopharyngeal carcinoma in the Taiwanese population. Int J Cancer. 1995 Sep 15;62(6):673–677. doi: 10.1002/ijc.2910620605. [DOI] [PubMed] [Google Scholar]
- Chen M. L., Tsai C. N., Liang C. L., Shu C. H., Huang C. R., Sulitzeanu D., Liu S. T., Chang Y. S. Cloning and characterization of the latent membrane protein (LMP) of a specific Epstein-Barr virus variant derived from the nasopharyngeal carcinoma in the Taiwanese population. Oncogene. 1992 Nov;7(11):2131–2140. [PubMed] [Google Scholar]
- Chen W. G., Chen Y. Y., Bacchi M. M., Bacchi C. E., Alvarenga M., Weiss L. M. Genotyping of Epstein-Barr virus in Brazilian Burkitt's lymphoma and reactive lymphoid tissue. Type A with a high prevalence of deletions within the latent membrane protein gene. Am J Pathol. 1996 Jan;148(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- Cheung S. T., Lo K. W., Leung S. F., Chan W. Y., Choi P. H., Johnson P. J., Lee J. C., Huang D. P. Prevalence of LMP1 deletion variant of Epstein-Barr virus in nasopharyngeal carcinoma and gastric tumors in Hong Kong. Int J Cancer. 1996 May 29;66(5):711–712. doi: 10.1002/(SICI)1097-0215(19960529)66:5<711::AID-IJC21>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Deamant F. D., Albújar P. F., Chen Y. Y., Weiss L. M. Epstein-Barr virus distribution in nonneoplastic lymph nodes. Mod Pathol. 1993 Nov;6(6):729–732. [PubMed] [Google Scholar]
- Fukayama M., Hayashi Y., Iwasaki Y., Chong J., Ooba T., Takizawa T., Koike M., Mizutani S., Miyaki M., Hirai K. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab Invest. 1994 Jul;71(1):73–81. [PubMed] [Google Scholar]
- Gratama J. W., Ernberg I. Molecular epidemiology of Epstein-Barr virus infection. Adv Cancer Res. 1995;67:197–255. [PubMed] [Google Scholar]
- Gulley M. L., Pulitzer D. R., Eagan P. A., Schneider B. G. Epstein-Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2 expression and p53 accumulation. Hum Pathol. 1996 Jan;27(1):20–27. doi: 10.1016/s0046-8177(96)90133-1. [DOI] [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Hu L. F., Chen F., Zheng X., Ernberg I., Cao S. L., Christensson B., Klein G., Winberg G. Clonability and tumorigenicity of human epithelial cells expressing the EBV encoded membrane protein LMP1. Oncogene. 1993 Jun;8(6):1575–1583. [PubMed] [Google Scholar]
- Hu L. F., Zabarovsky E. R., Chen F., Cao S. L., Ernberg I., Klein G., Winberg G. Isolation and sequencing of the Epstein-Barr virus BNLF-1 gene (LMP1) from a Chinese nasopharyngeal carcinoma. J Gen Virol. 1991 Oct;72(Pt 10):2399–2409. doi: 10.1099/0022-1317-72-10-2399. [DOI] [PubMed] [Google Scholar]
- Imai S., Koizumi S., Sugiura M., Tokunaga M., Uemura Y., Yamamoto N., Tanaka S., Sato E., Osato T. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9131–9135. doi: 10.1073/pnas.91.19.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye K. M., Izumi K. M., Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida Y., Miyauchi K., Takano Y. Gastric adenocarcinoma with differentiation to sarcomatous components associated with monoclonal Epstein-Barr virus infection and LMP-1 expression. Virchows Arch A Pathol Anat Histopathol. 1993;423(5):383–387. doi: 10.1007/BF01607151. [DOI] [PubMed] [Google Scholar]
- Kingma D. W., Weiss W. B., Jaffe E. S., Kumar S., Frekko K., Raffeld M. Epstein-Barr virus latent membrane protein-1 oncogene deletions: correlations with malignancy in Epstein-Barr virus--associated lymphoproliferative disorders and malignant lymphomas. Blood. 1996 Jul 1;88(1):242–251. [PubMed] [Google Scholar]
- Knecht H., Bachmann E., Brousset P., Rothenberger S., Einsele H., Lestou V. S., Delsol G., Bachmann F., Ambros P. F., Odermatt B. F. Mutational hot spots within the carboxy terminal region of the LMP1 oncogene of Epstein-Barr virus are frequent in lymphoproliferative disorders. Oncogene. 1995 Feb 2;10(3):523–528. [PubMed] [Google Scholar]
- Knecht H., Bachmann E., Brousset P., Sandvej K., Nadal D., Bachmann F., Odermatt B. F., Delsol G., Pallesen G. Deletions within the LMP1 oncogene of Epstein-Barr virus are clustered in Hodgkin's disease and identical to those observed in nasopharyngeal carcinoma. Blood. 1993 Nov 15;82(10):2937–2942. [PubMed] [Google Scholar]
- Knecht H., Raphaël M., McQuain C., Rothenberger S., Pihan G., Camilleri-Broët S., Bachmann E., Kershaw G. R., Ryan S., Kittler E. L. Deletion variants within the NF-kappa B activation domain of the LMP1 oncogene prevail in acquired immunodeficiency syndrome-related large cell lymphomas and human immunodeficiency virus-negative atypical lymphoproliferations. Blood. 1996 Feb 1;87(3):876–881. [PubMed] [Google Scholar]
- Kunimoto M., Tamura S., Tabata T., Yoshie O. One-step typing of Epstein-Barr virus by polymerase chain reaction: predominance of type 1 virus in Japan. J Gen Virol. 1992 Feb;73(Pt 2):455–461. doi: 10.1099/0022-1317-73-2-455. [DOI] [PubMed] [Google Scholar]
- Levine P. H., Stemmermann G., Lennette E. T., Hildesheim A., Shibata D., Nomura A. Elevated antibody titers to Epstein-Barr virus prior to the diagnosis of Epstein-Barr-virus-associated gastric adenocarcinoma. Int J Cancer. 1995 Mar 3;60(5):642–644. doi: 10.1002/ijc.2910600513. [DOI] [PubMed] [Google Scholar]
- Li S. N., Chang Y. S., Liu S. T. Effect of a 10-amino acid deletion on the oncogenic activity of latent membrane protein 1 of Epstein-Barr virus. Oncogene. 1996 May 16;12(10):2129–2135. [PubMed] [Google Scholar]
- Lin J. C., Lin S. C., De B. K., Chan W. P., Evatt B. L., Chan W. C. Precision of genotyping of Epstein-Barr virus by polymerase chain reaction using three gene loci (EBNA-2, EBNA-3C, and EBER): predominance of type A virus associated with Hodgkin's disease. Blood. 1993 Jun 15;81(12):3372–3381. [PubMed] [Google Scholar]
- Matsunou H., Konishi F., Hori H., Ikeda T., Sasaki K., Hirose Y., Yamamichi N. Characteristics of Epstein-Barr virus-associated gastric carcinoma with lymphoid stroma in Japan. Cancer. 1996 May 15;77(10):1998–2004. doi: 10.1002/(SICI)1097-0142(19960515)77:10<1998::AID-CNCR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Mosialos G., Birkenbach M., Yalamanchili R., VanArsdale T., Ware C., Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995 Feb 10;80(3):389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- Oda K., Tamaru J., Takenouchi T., Mikata A., Nunomura M., Saitoh N., Sarashina H., Nakajima N. Association of Epstein-Barr virus with gastric carcinoma with lymphoid stroma. Am J Pathol. 1993 Oct;143(4):1063–1071. [PMC free article] [PubMed] [Google Scholar]
- Ohfuji S., Osaki M., Tsujitani S., Ikeguchi M., Sairenji T., Ito H. Low frequency of apoptosis in Epstein-Barr virus-associated gastric carcinoma with lymphoid stroma. Int J Cancer. 1996 Dec 11;68(6):710–715. doi: 10.1002/1097-0215(19961211)68:6<710::aid-ijc2910680602>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Okan I., Wang Y., Chen F., Hu L. F., Imreh S., Klein G., Wiman K. G. The EBV-encoded LMP1 protein inhibits p53-triggered apoptosis but not growth arrest. Oncogene. 1995 Sep 21;11(6):1027–1031. [PubMed] [Google Scholar]
- Osato T., Imai S. Epstein-Barr virus and gastric carcinoma. Semin Cancer Biol. 1996 Aug;7(4):175–182. doi: 10.1006/scbi.1996.0024. [DOI] [PubMed] [Google Scholar]
- Ott G., Kirchner T., Müller-Hermelink H. K. Monoclonal Epstein-Barr virus genomes but lack of EBV-related protein expression in different types of gastric carcinoma. Histopathology. 1994 Oct;25(4):323–329. doi: 10.1111/j.1365-2559.1994.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Qiu K., Tomita Y., Hashimoto M., Ohsawa M., Kawano K., Wu D. M., Aozasa K. Epstein-Barr virus in gastric carcinoma in Suzhou, China and Osaka, Japan: association with clinico-pathologic factors and HLA-subtype. Int J Cancer. 1997 Apr 10;71(2):155–158. doi: 10.1002/(sici)1097-0215(19970410)71:2<155::aid-ijc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rogers B. B., Josephson S. L., Mak S. K. Detection of herpes simplex virus using the polymerase chain reaction followed by endonuclease cleavage. Am J Pathol. 1991 Jul;139(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Sandvej K., Gratama J. W., Munch M., Zhou X. G., Bolhuis R. L., Andresen B. S., Gregersen N., Hamilton-Dutoit S. Sequence analysis of the Epstein-Barr virus (EBV) latent membrane protein-1 gene and promoter region: identification of four variants among wild-type EBV isolates. Blood. 1997 Jul 1;90(1):323–330. [PubMed] [Google Scholar]
- Sandvej K., Peh S. C., Andresen B. S., Pallesen G. Identification of potential hot spots in the carboxy-terminal part of the Epstein-Barr virus (EBV) BNLF-1 gene in both malignant and benign EBV-associated diseases: high frequency of a 30-bp deletion in Malaysian and Danish peripheral T-cell lymphomas. Blood. 1994 Dec 15;84(12):4053–4060. [PubMed] [Google Scholar]
- Selves J., Bibeau F., Brousset P., Meggetto F., Mazerolles C., Voigt J. J., Pradere B., Chiotasso P., Delsol G. Epstein-Barr virus latent and replicative gene expression in gastric carcinoma. Histopathology. 1996 Feb;28(2):121–127. doi: 10.1046/j.1365-2559.1996.287333.x. [DOI] [PubMed] [Google Scholar]
- Sheibani K., Tubbs R. R. Enzyme immunohistochemistry: technical aspects. Semin Diagn Pathol. 1984 Nov;1(4):235–250. [PubMed] [Google Scholar]
- Shibata D., Weiss L. M. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992 Apr;140(4):769–774. [PMC free article] [PubMed] [Google Scholar]
- Sidagis J., Ueno K., Tokunaga M., Ohyama M., Eizuru Y. Molecular epidemiology of Epstein-Barr virus (EBV) in EBV-related malignancies. Int J Cancer. 1997 Jul 3;72(1):72–76. doi: 10.1002/(sici)1097-0215(19970703)72:1<72::aid-ijc11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Smir B. N., Hauke R. J., Bierman P. J., Gross T. G., d'Amore F., Anderson J. R., Greiner T. C. Molecular epidemiology of deletions and mutations of the latent membrane protein 1 oncogene of the Epstein-Barr virus in posttransplant lymphoproliferative disorders. Lab Invest. 1996 Oct;75(4):575–588. [PubMed] [Google Scholar]
- Sugiura M., Imai S., Tokunaga M., Koizumi S., Uchizawa M., Okamoto K., Osato T. Transcriptional analysis of Epstein-Barr virus gene expression in EBV-positive gastric carcinoma: unique viral latency in the tumour cells. Br J Cancer. 1996 Aug;74(4):625–631. doi: 10.1038/bjc.1996.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Land C. E., Uemura Y., Tokudome T., Tanaka S., Sato E. Epstein-Barr virus in gastric carcinoma. Am J Pathol. 1993 Nov;143(5):1250–1254. [PMC free article] [PubMed] [Google Scholar]
- Vasef M. A., Kamel O. W., Chen Y. Y., Medeiros L. J., Weiss L. M. Detection of Epstein-Barr virus in multiple sites involved by Hodgkin's disease. Am J Pathol. 1995 Nov;147(5):1408–1415. [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Chen Y. Y. Effects of different fixatives on detection of nucleic acids from paraffin-embedded tissues by in situ hybridization using oligonucleotide probes. J Histochem Cytochem. 1991 Sep;39(9):1237–1242. doi: 10.1177/39.9.1918942. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Movahed L. A., Chen Y. Y., Shin S. S., Stroup R. M., Bui N., Estess P., Bindl J. M. Detection of immunoglobulin light-chain mRNA in lymphoid tissues using a practical in situ hybridization method. Am J Pathol. 1990 Oct;137(4):979–988. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Tokunaga M., Uemura Y., Tanaka S., Shirahama H., Nakamura T., Land C. E., Sato E. Epstein-Barr virus and gastric remnant cancer. Cancer. 1994 Aug 1;74(3):805–809. doi: 10.1002/1097-0142(19940801)74:3<805::aid-cncr2820740304>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Yuen S. T., Chung L. P., Leung S. Y., Luk I. S., Chan S. Y., Ho J. In situ detection of Epstein-Barr virus in gastric and colorectal adenocarcinomas. Am J Surg Pathol. 1994 Nov;18(11):1158–1163. doi: 10.1097/00000478-199411000-00010. [DOI] [PubMed] [Google Scholar]