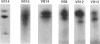Abstract
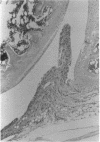
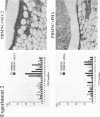
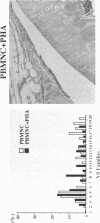
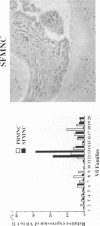
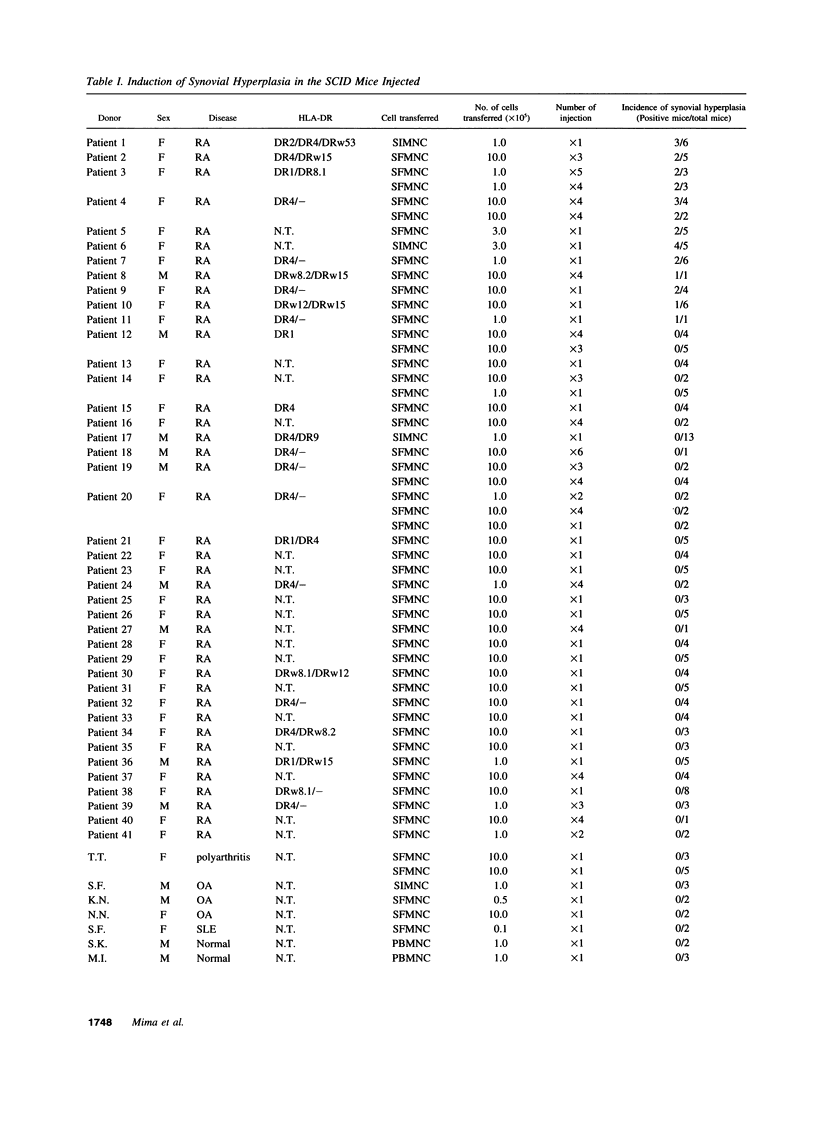
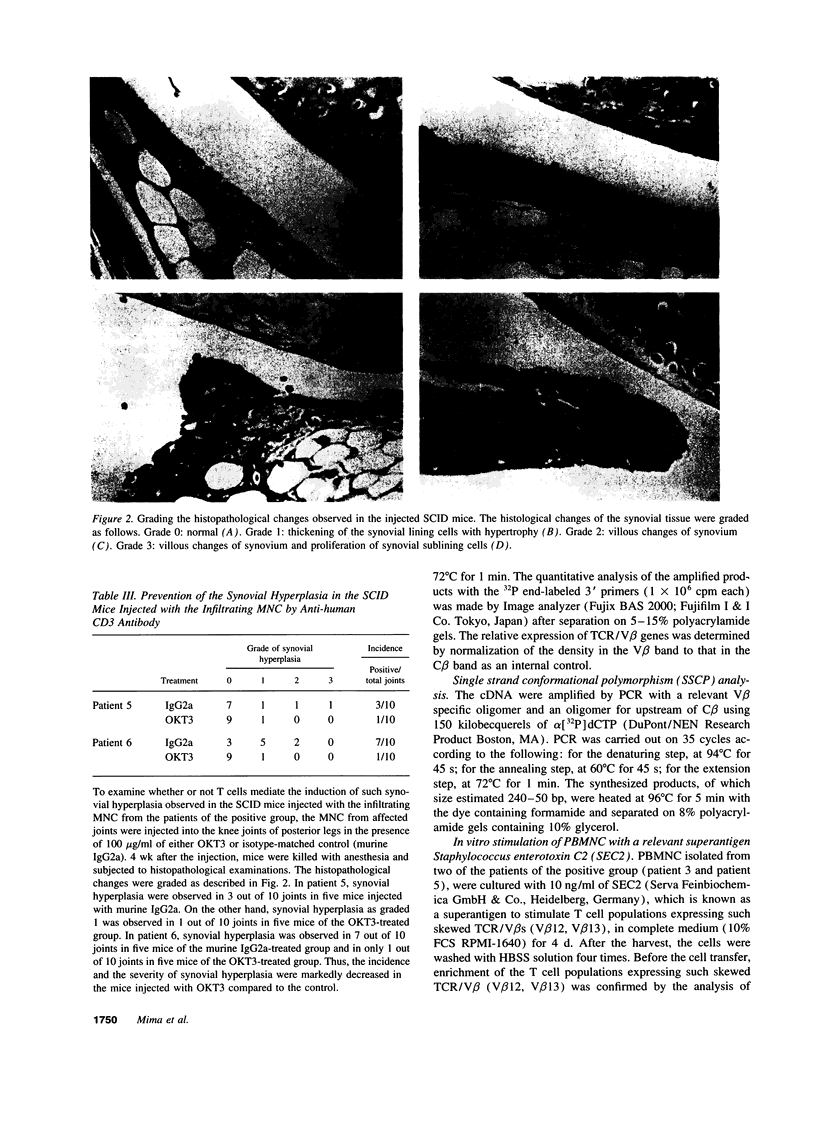
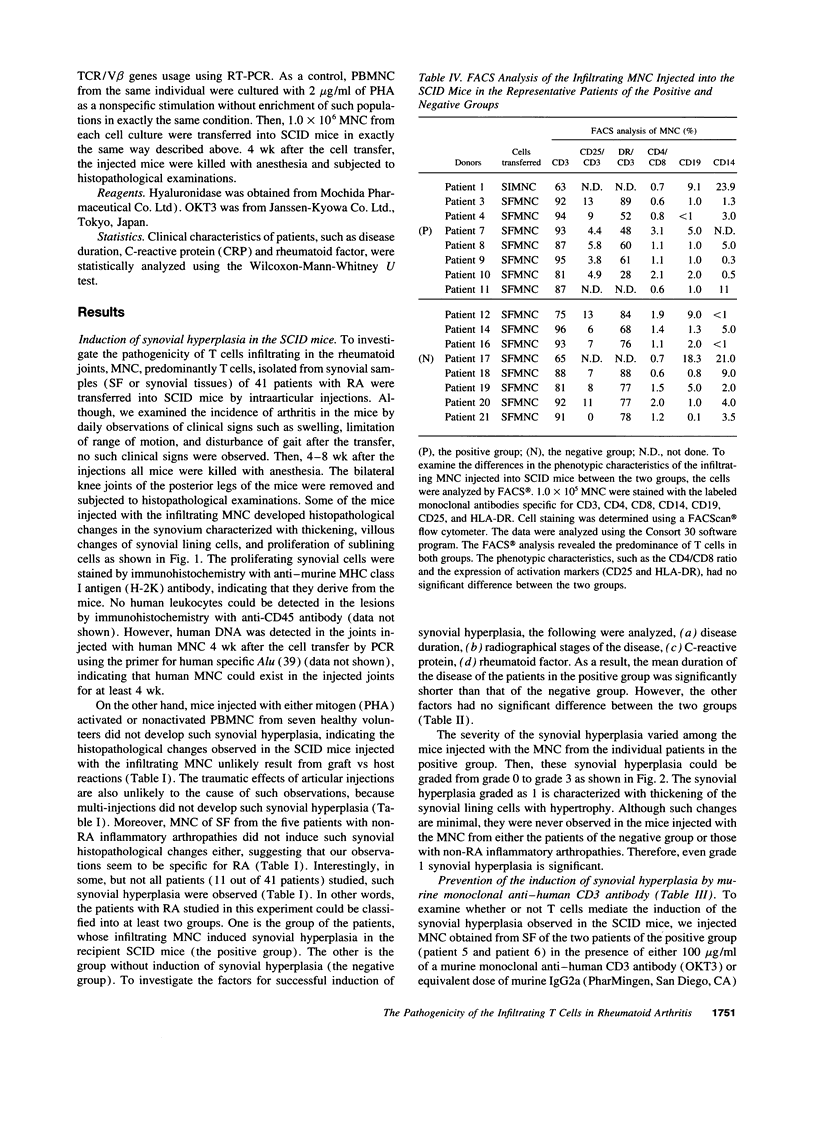
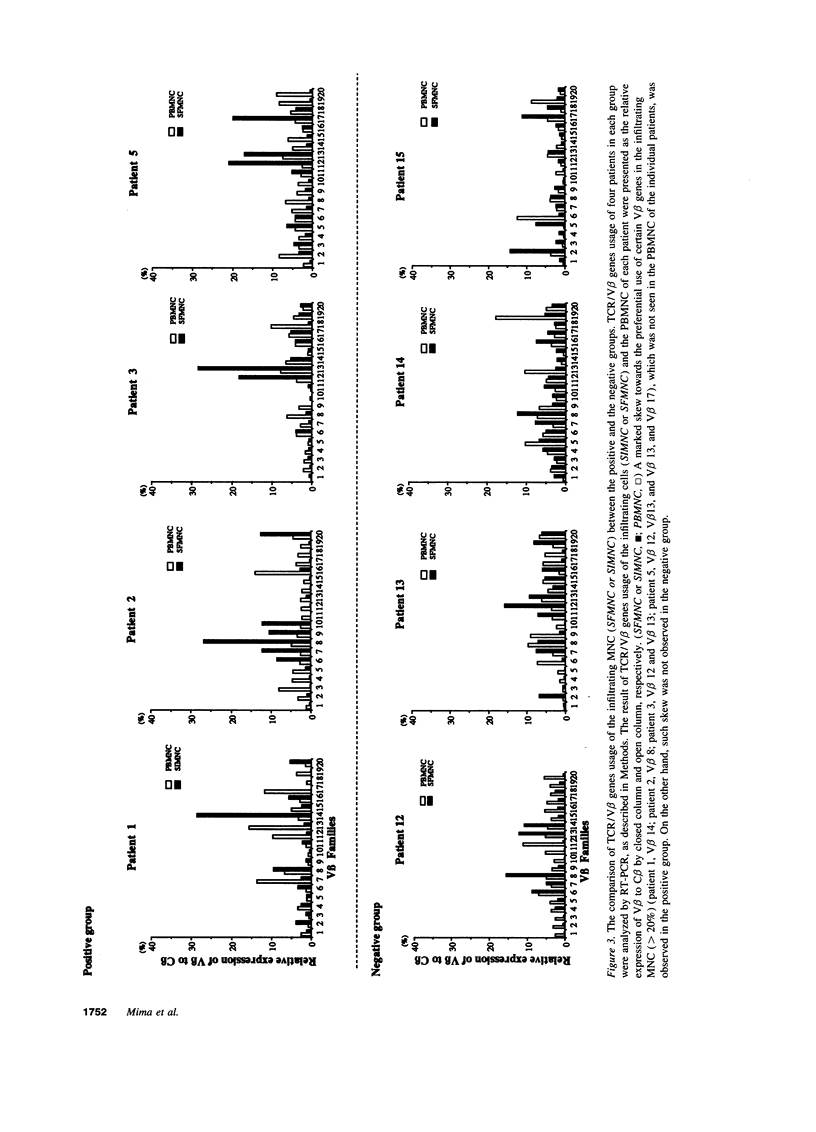
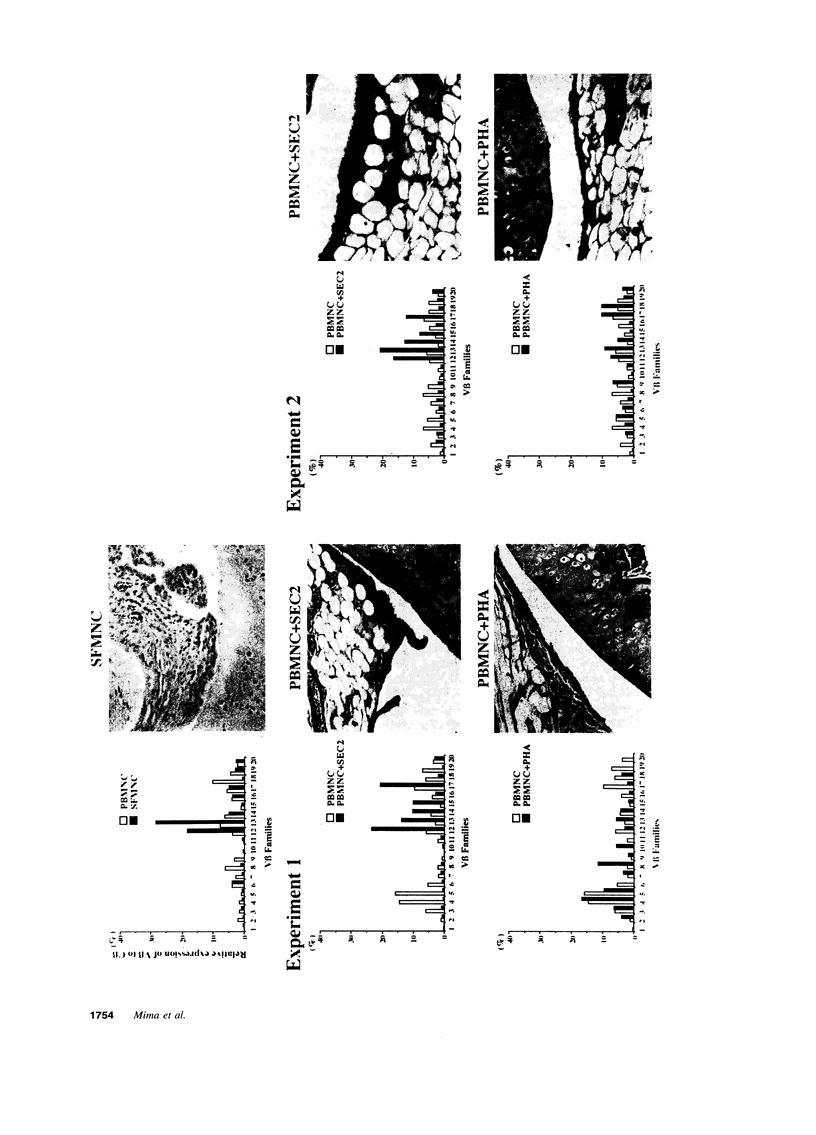
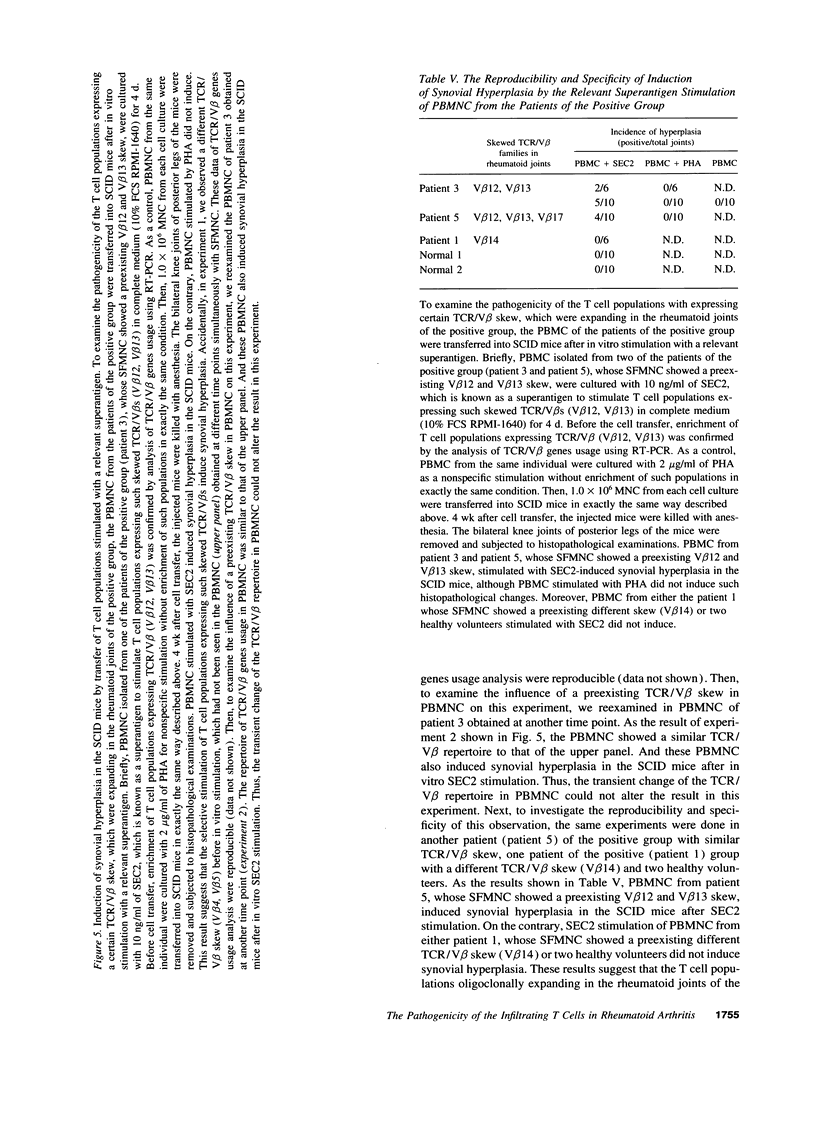
To investigate the pathogenicity of T cells infiltrating in the rheumatoid joints, mononuclear cells (MNC), predominantly T cells, isolated from either synovial fluid or synovial tissues of the patients with RA were transferred into severe combined immunodeficient (SCID) mice by intraarticular injections. According to our observations in this experimental system, patients with RA could be classified into at least two groups. In one group of patients, the infiltrating MNC induced synovial hyperplasia in the recipient SCID mice (the positive group). Whereas, in the other group no synovial hyperplasia was observed (the negative group). The induction of synovial hyperplasia observed in the positive group was prevented by an anti-human CD3 antibody (OKT3), indicating T cell mediation. Analysis of T cell receptor (TCR) V beta usage by reverse transcriptase polymerase chain reaction in the infiltrating MNC transferred into SCID mice revealed a marked skew towards the preferential use of certain V beta genes, which was not seen in the peripheral blood MNC, in only the positive group. The patterns of TCR/V beta skew were not uniform among the patients. The analysis of the PCR-amplified genes of such skewed TCR/ V beta by single strand conformational polymorphism showed distinct bands, indicating that the T cell populations expanding in rheumatoid joints of the positive group were oligoclonal. Furthermore, the enrichment of the T cell populations expressing such skewed TCR/V beta by in vitro stimulation of peripheral blood MNC of the patients with the relevant superantigen enabled the induction of synovial hyperplasia in the SCID mice. These results suggest that the pathogenic T cells could be activated locally in rheumatoid joints by certain antigens in some, but not in all patients with RA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsen T. G., Froland S. S., Natvig J. B., Pahle J. Elution and characterization of lymphocytes from rheumatoid inflammatory tissue. Scand J Immunol. 1975;4(8):823–830. doi: 10.1111/j.1365-3083.1975.tb03723.x. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Chiocchia G., Boissier M. C., Fournier C. Therapy against murine collagen-induced arthritis with T cell receptor V beta-specific antibodies. Eur J Immunol. 1991 Dec;21(12):2899–2905. doi: 10.1002/eji.1830211202. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Londei M., Leech Z., Brennan F., Savill C., Maini R. N. Analysis of T cell clones in rheumatoid arthritis. Springer Semin Immunopathol. 1988;10(2-3):157–167. doi: 10.1007/BF01857221. [DOI] [PubMed] [Google Scholar]
- Funkhouser S. W., Concannon P., Charmley P., Vredevoe D. L., Hood L. Differences in T cell receptor restriction fragment length polymorphisms in patients with rheumatoid arthritis. Arthritis Rheum. 1992 Apr;35(4):465–471. doi: 10.1002/art.1780350417. [DOI] [PubMed] [Google Scholar]
- Goldschmidt T. J., Holmdahl R. Anti-T cell receptor antibody treatment of rats with established autologous collagen-induced arthritis: suppression of arthritis without reduction of anti-type II collagen autoantibody levels. Eur J Immunol. 1991 May;21(5):1327–1330. doi: 10.1002/eji.1830210536. [DOI] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Gudmundsson S., Rönnelid J., Karlsson-Parra A., Lysholm J., Gudbjörnsson B., Widenfalk B., Janson C. H., Klareskog L. T-cell receptor V-gene usage in synovial fluid and synovial tissue from RA patients. Scand J Immunol. 1992 Nov;36(5):681–688. doi: 10.1111/j.1365-3083.1992.tb03128.x. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Klareskog L., Rubin K., Larsson E., Wigzell H. T lymphocytes in collagen II-induced arthritis in mice. Characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand J Immunol. 1985 Sep;22(3):295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- Horneff G., Burmester G. R., Emmrich F., Kalden J. R. Treatment of rheumatoid arthritis with an anti-CD4 monoclonal antibody. Arthritis Rheum. 1991 Feb;34(2):129–140. doi: 10.1002/art.1780340202. [DOI] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs J. D., Watts R. A., Hazleman B. L., Hale G., Keogan M. T., Cobbold S. P., Waldmann H. Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet. 1992 Sep 26;340(8822):748–752. doi: 10.1016/0140-6736(92)92294-p. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Tosu M., Yoshida E., Takiguchi M., Sato K., Kitajima I., Nishioka K., Yamamoto K., Takeda T., Hatanaka M. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-I. Science. 1991 Aug 30;253(5023):1026–1028. doi: 10.1126/science.1887217. [DOI] [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Kakimoto K., Katsuki M., Hirofuji T., Iwata H., Koga T. Isolation of T cell line capable of protecting mice against collagen-induced arthritis. J Immunol. 1988 Jan 1;140(1):78–83. [PubMed] [Google Scholar]
- Karsh J., Klippel J. H., Plotz P. H., Decker J. L., Wright D. G., Flye M. W. Lymphapheresis in rheumatoid arthritis. A randomized trial. Arthritis Rheum. 1981 Jul;24(7):867–873. doi: 10.1002/art.1780240701. [DOI] [PubMed] [Google Scholar]
- Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991 Dec;10(13):4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Miltenburg A. M., van Laar J. M., Daha M. R., de Vries R. R., van den Elsen P. J., Breedveld F. C. Dominant T-cell receptor beta-chain gene rearrangements indicate clonal expansion in the rheumatoid joint. Scand J Immunol. 1990 Jan;31(1):121–126. doi: 10.1111/j.1365-3083.1990.tb02750.x. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Ledbetter S. A., Corbo L., Victoria M. F., Ramírez-Solis R., Webster T. D., Ledbetter D. H., Caskey C. T. Alu polymerase chain reaction: a method for rapid isolation of human-specific sequences from complex DNA sources. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6686–6690. doi: 10.1073/pnas.86.17.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G. T., Byers P., Seyfried C., Healey L. A., Wilske K. R., Stage D., Nepom B. S. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989 Jan;32(1):15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- Nüsslein H. G., Herbst M., Manger B. J., Gramatzki M., Burmester G. R., Fritz H., Sauer R., Kalden J. R. Total lymphoid irradiation in patients with refractory rheumatoid arthritis. Arthritis Rheum. 1985 Nov;28(11):1205–1210. doi: 10.1002/art.1780281103. [DOI] [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack U., Kuhn H., Ermann J., Kinne R. W., Vogt S., Jungmichel D., Emmrich F. Synovial tissue implants from patients with rheumatoid arthritis cause cartilage destruction in knee joints of SCID.bg mice. J Rheumatol. 1994 Jan;21(1):10–16. [PubMed] [Google Scholar]
- Saeki Y., Mima T., Sakoda S., Fujimura H., Arita N., Nomura T., Kishimoto T. Transfer of multiple sclerosis into severe combined immunodeficiency mice by mononuclear cells from cerebrospinal fluid of the patients. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6157–6161. doi: 10.1073/pnas.89.13.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroky J. B., Yocum D. E., Wilder R. L., Klippel J. H. Experimental basis of innovative therapies of rheumatoid arthritis. Concepts Immunopathol. 1989;7:106–144. [PubMed] [Google Scholar]
- Smith T. J., Terada N., Robinson C. C., Gelfand E. W. Acute infectious mononucleosis stimulates the selective expression/expansion of V beta 6.1-3 and V beta 7 T cells. Blood. 1993 Mar 15;81(6):1521–1526. [PubMed] [Google Scholar]
- Sottini A., Imberti L., Gorla R., Cattaneo R., Primi D. Restricted expression of T cell receptor V beta but not V alpha genes in rheumatoid arthritis. Eur J Immunol. 1991 Feb;21(2):461–466. doi: 10.1002/eji.1830210231. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida T., Yonaha F., Maeda T., Tanabe E., Koike T., Tomioka H., Yoshida S. T cell receptor repertoire of infiltrating T cells in lips of Sjögren's syndrome patients. J Clin Invest. 1992 Feb;89(2):681–685. doi: 10.1172/JCI115635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Uematsu Y., Wege H., Straus A., Ott M., Bannwarth W., Lanchbury J., Panayi G., Steinmetz M. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8534–8538. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Williams W. V., Fang Q., Demarco D., VonFeldt J., Zurier R. B., Weiner D. B. Restricted heterogeneity of T cell receptor transcripts in rheumatoid synovium. J Clin Invest. 1992 Aug;90(2):326–333. doi: 10.1172/JCI115866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordsworth B. P., Lanchbury J. S., Sakkas L. I., Welsh K. I., Panayi G. S., Bell J. I. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Sakoda H., Nakajima T., Kato T., Okubo M., Dohi M., Mizushima Y., Ito K., Nishioka K. Accumulation of multiple T cell clonotypes in the synovial lesions of patients with rheumatoid arthritis revealed by a novel clonality analysis. Int Immunol. 1992 Nov;4(11):1219–1223. doi: 10.1093/intimm/4.11.1219. [DOI] [PubMed] [Google Scholar]