Abstract
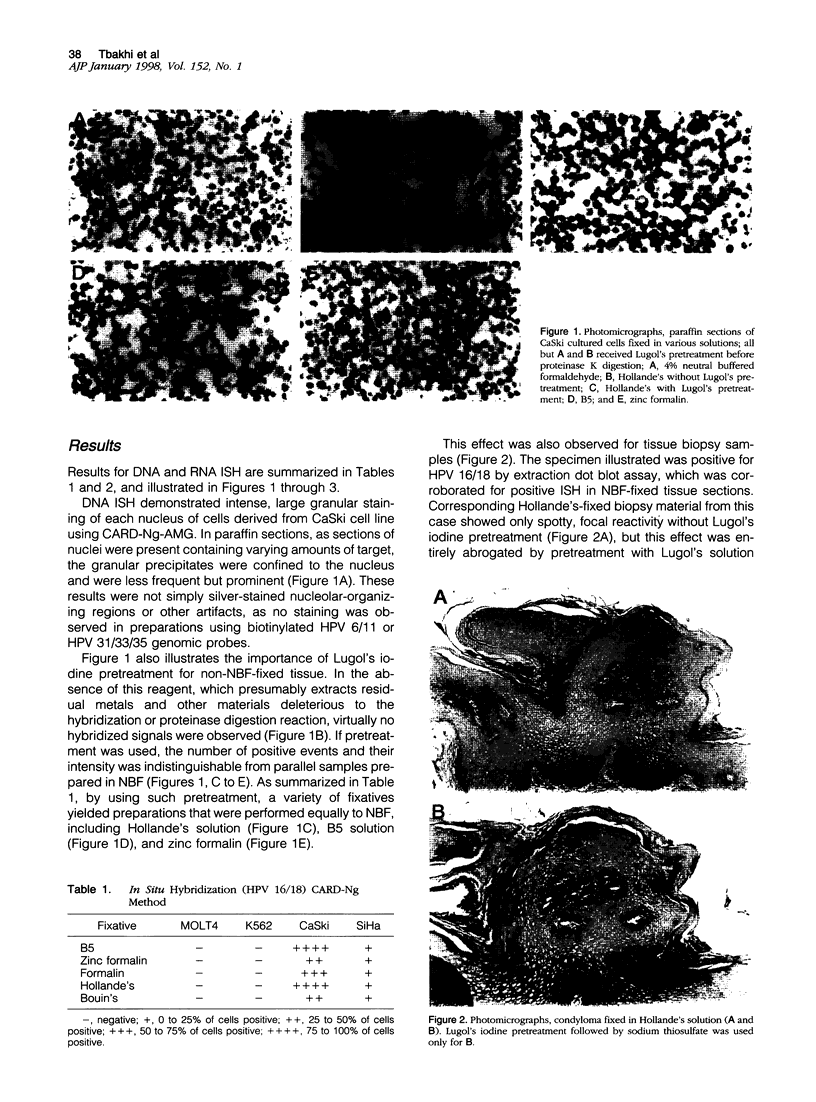
Neutral buffered formalin (NBF) (4% neutral buffered formaldehyde) has been advocated by most investigators as the primary fixative of choice for in situ hybridization (ISH), and specific anecdotal cautions interdicting the use of precipitating fixatives, which otherwise may offer certain advantages such as superior nuclear detail, are common. Few systematic studies addressing ISH fixation conditions have been published. We reasoned that heavy metals present in some precipitating fixatives may compromise duplex formation during ISH. Cell lines containing known viral gene content (CaSki, 200 to 600 human papilloma virus 16 copies/cell, and SiHa, 1 to 2 human papilloma virus 16 copies/cell) and two negative cell lines (K562 and MOLT 4) were expanded to >10(10) and pellets fixed in NBF, zinc formalin, B5, and Bouin's and Hollande's solutions, and subjected to DNA ISH using biotinylated genomic probes. Ten tissue biopsies fixed in both Hollande's and NBF solutions were also evaluated for human papilloma virus content using DNA ISH. Additionally, 17 cases of Hodgkin's disease fixed in B5 and formalin were compared for Epstein-Barr encoded RNA detection using RNA ISH with fluorescein isothiocyanate-labeled oligonucleotides. Catalyzed reporter deposition combined with Streptavidin-Nanogold staining and silver acetate autometallography (Catalyzed reporter deposition-Ng-autometallography ISH) and a conventional indirect alkaline phosphatase method were used for detection for both DNA and RNA. Contaminating heavy metals entrapped in fixed tissues were removed by two exposures to Lugol's iodine. Results for both DNA and RNA ISH comparing B5 and NBF fixatives were virtually identical. Hollande's, Bouin's, B5, and zinc formalin fixed tissue showed results indistinguishable from NBF fixed tissue in DNA ISH. Precipitating fixatives such as B5 and Hollande's solution may be used for DNA and RNA ISH under appropriate conditions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Angerer R. C. Localization of mRNAs by in situ hybridization. Methods Cell Biol. 1991;35:37–71. doi: 10.1016/s0091-679x(08)60568-3. [DOI] [PubMed] [Google Scholar]
- Bobrow M. N., Harris T. D., Shaughnessy K. J., Litt G. J. Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J Immunol Methods. 1989 Dec 20;125(1-2):279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- Bobrow M. N., Shaughnessy K. J., Litt G. J. Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. J Immunol Methods. 1991 Mar 1;137(1):103–112. doi: 10.1016/0022-1759(91)90399-z. [DOI] [PubMed] [Google Scholar]
- Dubeau L., Chandler L. A., Gralow J. R., Nichols P. W., Jones P. A. Southern blot analysis of DNA extracted from formalin-fixed pathology specimens. Cancer Res. 1986 Jun;46(6):2964–2969. [PubMed] [Google Scholar]
- Friedl F., Kimura I., Osato T., Ito Y. Studies on a new human cell line (SiHa) derived from carcinoma of uterus. I. Its establishment and morphology. Proc Soc Exp Biol Med. 1970 Nov;135(2):543–545. doi: 10.3181/00379727-135-35091a. [DOI] [PubMed] [Google Scholar]
- Goelz S. E., Hamilton S. R., Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985 Jul 16;130(1):118–126. doi: 10.1016/0006-291x(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Hacker G. W., Graf A. H., Hauser-Kronberger C., Wirnsberger G., Schiechl A., Bernatzky G., Wittauer U., Su H. C., Adam H., Thurner J. Application of silver acetate autometallography and gold-silver staining methods for in situ DNA hybridization. Chin Med J (Engl) 1993 Feb;106(2):83–92. [PubMed] [Google Scholar]
- Hainfeld J. F., Furuya F. R. A 1.4-nm gold cluster covalently attached to antibodies improves immunolabeling. J Histochem Cytochem. 1992 Feb;40(2):177–184. doi: 10.1177/40.2.1552162. [DOI] [PubMed] [Google Scholar]
- Kerstens H. M., Poddighe P. J., Hanselaar A. G. A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J Histochem Cytochem. 1995 Apr;43(4):347–352. doi: 10.1177/43.4.7897179. [DOI] [PubMed] [Google Scholar]
- McAllister H. A., Rock D. L. Comparative usefulness of tissue fixatives for in situ viral nucleic acid hybridization. J Histochem Cytochem. 1985 Oct;33(10):1026–1032. doi: 10.1177/33.10.2995481. [DOI] [PubMed] [Google Scholar]
- Mincheva A., Gissmann L., zur Hausen H. Chromosomal integration sites of human papillomavirus DNA in three cervical cancer cell lines mapped by in situ hybridization. Med Microbiol Immunol. 1987;176(5):245–256. doi: 10.1007/BF00190531. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Richart R. M. Buffered formalin is the superior fixative for the detection of HPV DNA by in situ hybridization analysis. Am J Pathol. 1989 Apr;134(4):837–842. [PMC free article] [PubMed] [Google Scholar]
- Nuovo G. J., Silverstein S. J. Comparison of formalin, buffered formalin, and Bouin's fixation on the detection of human papillomavirus deoxyribonucleic acid from genital lesions. Lab Invest. 1988 Nov;59(5):720–724. [PubMed] [Google Scholar]
- Pattillo R. A., Hussa R. O., Story M. T., Ruckert A. C., Shalaby M. R., Mattingly R. F. Tumor antigen and human chorionic gonadotropin in CaSki cells: a new epidermoid cervical cancer cell line. Science. 1977 Jun 24;196(4297):1456–1458. doi: 10.1126/science.867042. [DOI] [PubMed] [Google Scholar]
- Raap A. K., van de Corput M. P., Vervenne R. A., van Gijlswijk R. P., Tanke H. J., Wiegant J. Ultra-sensitive FISH using peroxidase-mediated deposition of biotin- or fluorochrome tyramides. Hum Mol Genet. 1995 Apr;4(4):529–534. doi: 10.1093/hmg/4.4.529. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Broker T. R. In situ hybridization detection of human papillomavirus DNAs and messenger RNAs in genital condylomas and a cervical carcinoma. Hum Pathol. 1986 Dec;17(12):1250–1258. doi: 10.1016/s0046-8177(86)80569-x. [DOI] [PubMed] [Google Scholar]
- Stoler M. H. In situ hybridization. Clin Lab Med. 1990 Mar;10(1):215–236. [PubMed] [Google Scholar]
- Stoler M. H., Rhodes C. R., Whitbeck A., Wolinsky S. M., Chow L. T., Broker T. R. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992 Feb;23(2):117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- Zehbe I., Hacker G. W., Su H., Hauser-Kronberger C., Hainfeld J. F., Tubbs R. Sensitive in situ hybridization with catalyzed reporter deposition, streptavidin-Nanogold, and silver acetate autometallography: detection of single-copy human papillomavirus. Am J Pathol. 1997 May;150(5):1553–1561. [PMC free article] [PubMed] [Google Scholar]