Abstract
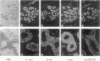
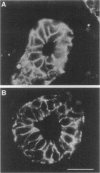
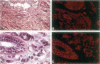
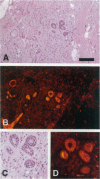
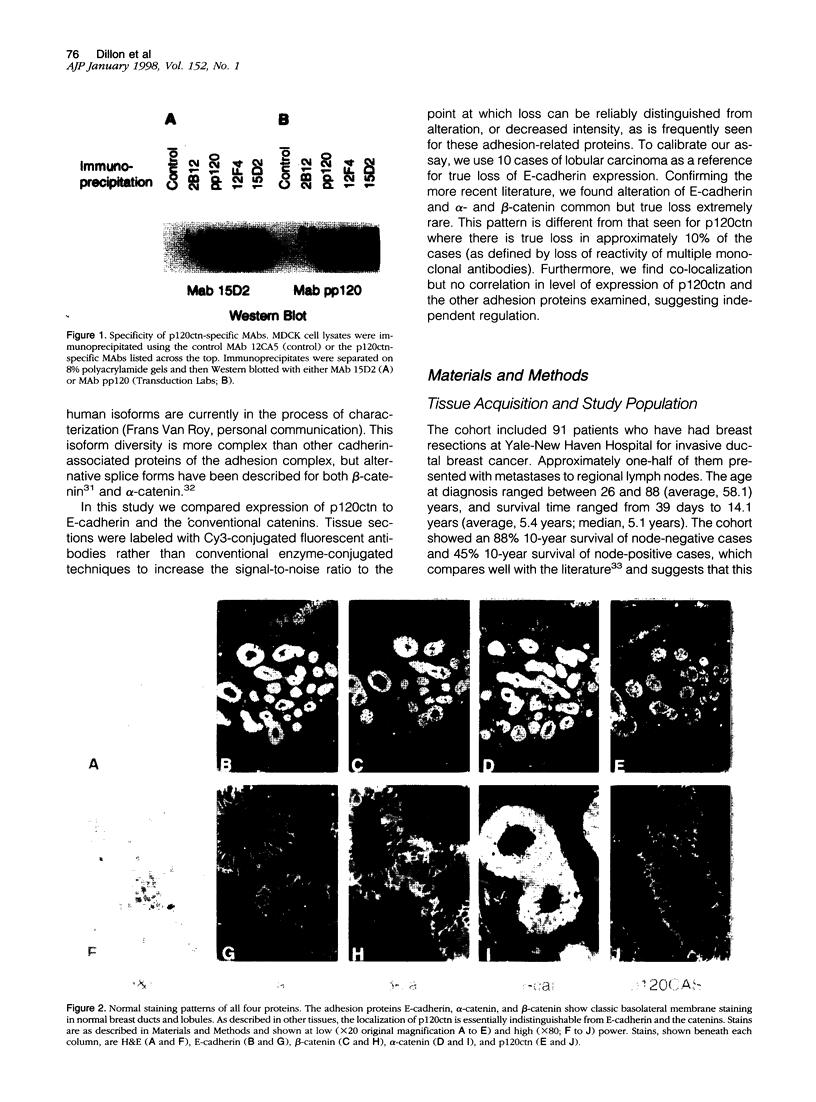
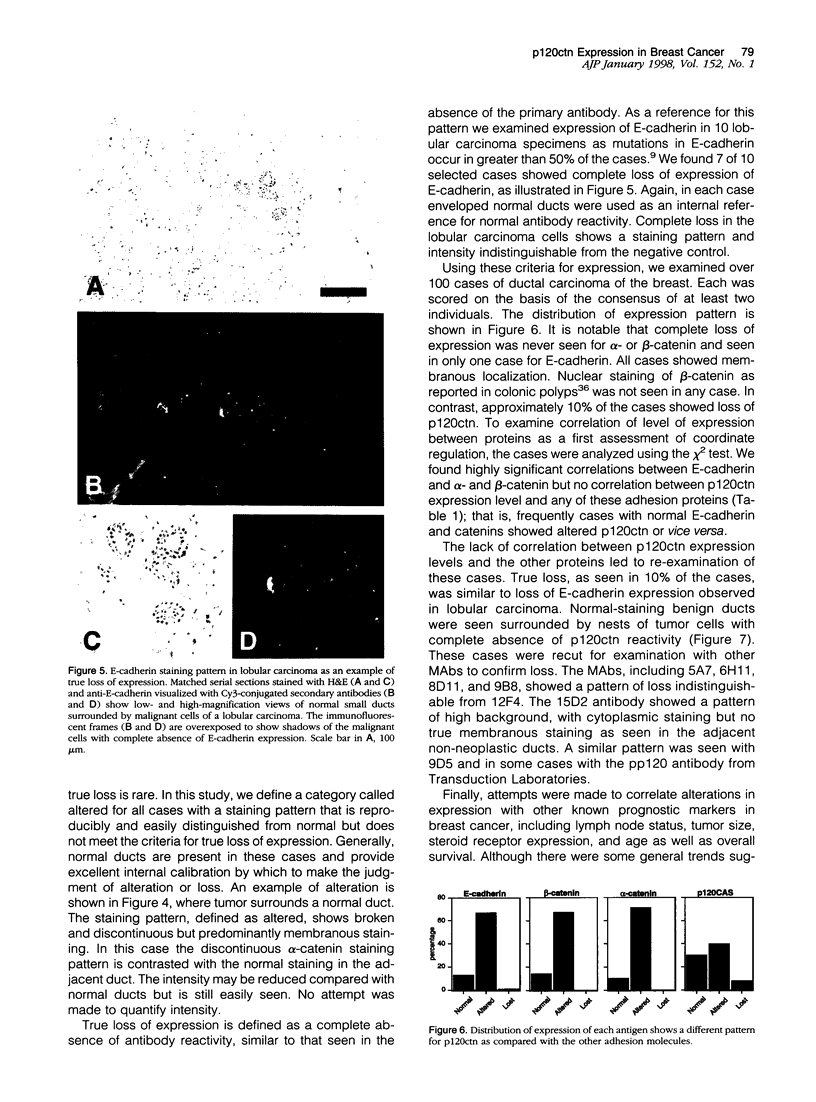
Several studies have reported loss or alteration of expression of E-cadherin in breast cancer and more recently changes in levels of expression of the catenins. We used immunofluorescence to examine E-cadherin, alpha-catenin, beta-catenin, and p120ctn (formerly p120CAS) expression in 91 cases of invasive ductal carcinoma. As expected, all four proteins co-localize to the junctional regions of the cells. Although nuclear localization has been described for beta-catenin in colonic polyps, no examples were found in these breast cancer cases. We found that, although alteration is common in the catenins and E-cadherin, complete loss, as exemplified by E-cadherin in lobular carcinoma (where E-cadherin is frequently mutated), is rarely seen. In contrast, the catenin-related protein p120ctn shows an expression pattern that is significantly unrelated to the other catenins (or E-cadherin), including complete loss of expression in approximately 10% of the cases. No statistically significant correlations with traditional prognostic indicators were observed with any of these proteins. We conclude 1) that expression of E-cadherin and alpha- and beta-catenin are generally retained at the membrane although frequently reduced or altered, 2) that complete loss of p120ctn expression is seen in approximately 10% of the cases, and 3) that there is a significant correlation in the expression of E-cadherin and the catenins but no correlation between these molecules and p120ctn, suggesting an absence of coordinate regulation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens J. The role of cell adhesion molecules in cancer invasion and metastasis. Breast Cancer Res Treat. 1993;24(3):175–184. doi: 10.1007/BF01833258. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997 Feb;9(1):99–108. doi: 10.1016/s0955-0674(97)80158-5. [DOI] [PubMed] [Google Scholar]
- Berx G., Cleton-Jansen A. M., Strumane K., de Leeuw W. J., Nollet F., van Roy F., Cornelisse C. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996 Nov 7;13(9):1919–1925. [PubMed] [Google Scholar]
- Candidus S., Bischoff P., Becker K. F., Höfler H. No evidence for mutations in the alpha- and beta-catenin genes in human gastric and breast carcinomas. Cancer Res. 1996 Jan 1;56(1):49–52. [PubMed] [Google Scholar]
- Cowin P., Burke B. Cytoskeleton-membrane interactions. Curr Opin Cell Biol. 1996 Feb;8(1):56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- Daniel J. M., Reynolds A. B. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol Cell Biol. 1995 Sep;15(9):4819–4824. doi: 10.1128/mcb.15.9.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan W. L. Tumor-related prognostic factors for breast cancer. CA Cancer J Clin. 1997 Jan-Feb;47(1):28–51. doi: 10.3322/canjclin.47.1.28. [DOI] [PubMed] [Google Scholar]
- Downing J. R., Reynolds A. B. PDGF, CSF-1, and EGF induce tyrosine phosphorylation of p120, a pp60src transformation-associated substrate. Oncogene. 1991 Apr;6(4):607–613. [PubMed] [Google Scholar]
- Gamallo C., Palacios J., Suarez A., Pizarro A., Navarro P., Quintanilla M., Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993 Apr;142(4):987–993. [PMC free article] [PubMed] [Google Scholar]
- Hashizume R., Koizumi H., Ihara A., Ohta T., Uchikoshi T. Expression of beta-catenin in normal breast tissue and breast carcinoma: a comparative study with epithelial cadherin and alpha-catenin. Histopathology. 1996 Aug;29(2):139–146. doi: 10.1046/j.1365-2559.1996.d01-499.x. [DOI] [PubMed] [Google Scholar]
- Hiscox S., Jiang W. G. Expression of E-cadherin, alpha, beta and gamma-catenin in human colorectal cancer. Anticancer Res. 1997 Mar-Apr;17(2B):1349–1354. [PubMed] [Google Scholar]
- Jiang W. G. E-cadherin and its associated protein catenins, cancer invasion and metastasis. Br J Surg. 1996 Apr;83(4):437–446. doi: 10.1002/bjs.1800830404. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Oda T., Tsuda H., Ochiai A., Hirohashi S. Point mutation of the E-cadherin gene in invasive lobular carcinoma of the breast. Jpn J Cancer Res. 1994 Oct;85(10):1035–1039. doi: 10.1111/j.1349-7006.1994.tb02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Parsons J. T. Tyrosine phosphorylation of a 120-kilodalton pp60src substrate upon epidermal growth factor and platelet-derived growth factor receptor stimulation and in polyomavirus middle-T-antigen-transformed cells. Mol Cell Biol. 1991 Feb;11(2):713–720. doi: 10.1128/mcb.11.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch M. S., Clark G. J., Der C. J., Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol. 1995 Jul;130(2):461–471. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y. Y., Reynolds A. B. Identification of murine p120 isoforms and heterogeneous expression of p120cas isoforms in human tumor cell lines. Cancer Res. 1996 Jun 1;56(11):2633–2640. [PubMed] [Google Scholar]
- Moll R., Mitze M., Frixen U. H., Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993 Dec;143(6):1731–1742. [PMC free article] [PubMed] [Google Scholar]
- Nollet F., Berx G., Molemans F., van Roy F. Genomic organization of the human beta-catenin gene (CTNNB1). Genomics. 1996 Mar 15;32(3):413–424. doi: 10.1006/geno.1996.0136. [DOI] [PubMed] [Google Scholar]
- Norton A. J., Jordan S., Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994 Aug;173(4):371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- Ochiai A., Akimoto S., Shimoyama Y., Nagafuchi A., Tsukita S., Hirohashi S. Frequent loss of alpha catenin expression in scirrhous carcinomas with scattered cell growth. Jpn J Cancer Res. 1994 Mar;85(3):266–273. doi: 10.1111/j.1349-7006.1994.tb02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka H., Shiozaki H., Kobayashi K., Inoue M., Tahara H., Kobayashi T., Takatsuka Y., Matsuyoshi N., Hirano S., Takeichi M. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993 Apr 1;53(7):1696–1701. [PubMed] [Google Scholar]
- Papkoff J. Regulation of complexed and free catenin pools by distinct mechanisms. Differential effects of Wnt-1 and v-Src. J Biol Chem. 1997 Feb 14;272(7):4536–4543. [PubMed] [Google Scholar]
- Peifer M., Berg S., Reynolds A. B. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994 Mar 11;76(5):789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Peifer M. Beta-catenin as oncogene: the smoking gun. Science. 1997 Mar 21;275(5307):1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- Pierceall W. E., Woodard A. S., Morrow J. S., Rimm D., Fearon E. R. Frequent alterations in E-cadherin and alpha- and beta-catenin expression in human breast cancer cell lines. Oncogene. 1995 Oct 5;11(7):1319–1326. [PubMed] [Google Scholar]
- Reynolds A. B., Daniel J., McCrea P. D., Wheelock M. J., Wu J., Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994 Dec;14(12):8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. B., Kanner S. B., Wang H. C., Parsons J. T. Stable association of activated pp60src with two tyrosine-phosphorylated cellular proteins. Mol Cell Biol. 1989 Sep;9(9):3951–3958. doi: 10.1128/mcb.9.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm D. L., Kebriaei P., Morrow J. S. Molecular cloning reveals alternative splice forms of human alpha(E)-catenin. Biochem Biophys Res Commun. 1994 Sep 30;203(3):1691–1699. doi: 10.1006/bbrc.1994.2381. [DOI] [PubMed] [Google Scholar]
- Rimm D. L., Sinard J. H., Morrow J. S. Reduced alpha-catenin and E-cadherin expression in breast cancer. Lab Invest. 1995 May;72(5):506–512. [PubMed] [Google Scholar]
- Shibamoto S., Hayakawa M., Takeuchi K., Hori T., Miyazawa K., Kitamura N., Johnson K. R., Wheelock M. J., Matsuyoshi N., Takeichi M. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J Cell Biol. 1995 Mar;128(5):949–957. doi: 10.1083/jcb.128.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazui T., Schalken J. A., Giroldi L. A., Jansen C. F., Akaza H., Koiso K., Debruyne F. M., Bringuier P. P. Prognostic value of cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120cas) in bladder tumors. Cancer Res. 1996 Sep 15;56(18):4154–4158. [PubMed] [Google Scholar]
- Siitonen S. M., Kononen J. T., Helin H. J., Rantala I. S., Holli K. A., Isola J. J. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol. 1996 Apr;105(4):394–402. doi: 10.1093/ajcp/105.4.394. [DOI] [PubMed] [Google Scholar]
- Skoudy A., Gomez S., Fabre M., Garcia de Herreros A. p120-catenin expression in human colorectal cancer. Int J Cancer. 1996 Sep 27;68(1):14–20. doi: 10.1002/(SICI)1097-0215(19960927)68:1<14::AID-IJC3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Sommers C. L., Gelmann E. P., Kemler R., Cowin P., Byers S. W. Alterations in beta-catenin phosphorylation and plakoglobin expression in human breast cancer cells. Cancer Res. 1994 Jul 1;54(13):3544–3552. [PubMed] [Google Scholar]
- Staddon J. M., Smales C., Schulze C., Esch F. S., Rubin L. L. p120, a p120-related protein (p100), and the cadherin/catenin complex. J Cell Biol. 1995 Jul;130(2):369–381. doi: 10.1083/jcb.130.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995 Oct;7(5):619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Valizadeh A., Karayiannakis A. J., el-Hariry I., Kmiot W., Pignatelli M. Expression of E-cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120) in colorectal polyps. Am J Pathol. 1997 Jun;150(6):1977–1984. [PMC free article] [PubMed] [Google Scholar]
- Zschiesche W., Schönborn I., Behrens J., Herrenknecht K., Hartveit F., Lilleng P., Birchmeier W. Expression of E-cadherin and catenins in invasive mammary carcinomas. Anticancer Res. 1997 Jan-Feb;17(1B):561–567. [PubMed] [Google Scholar]