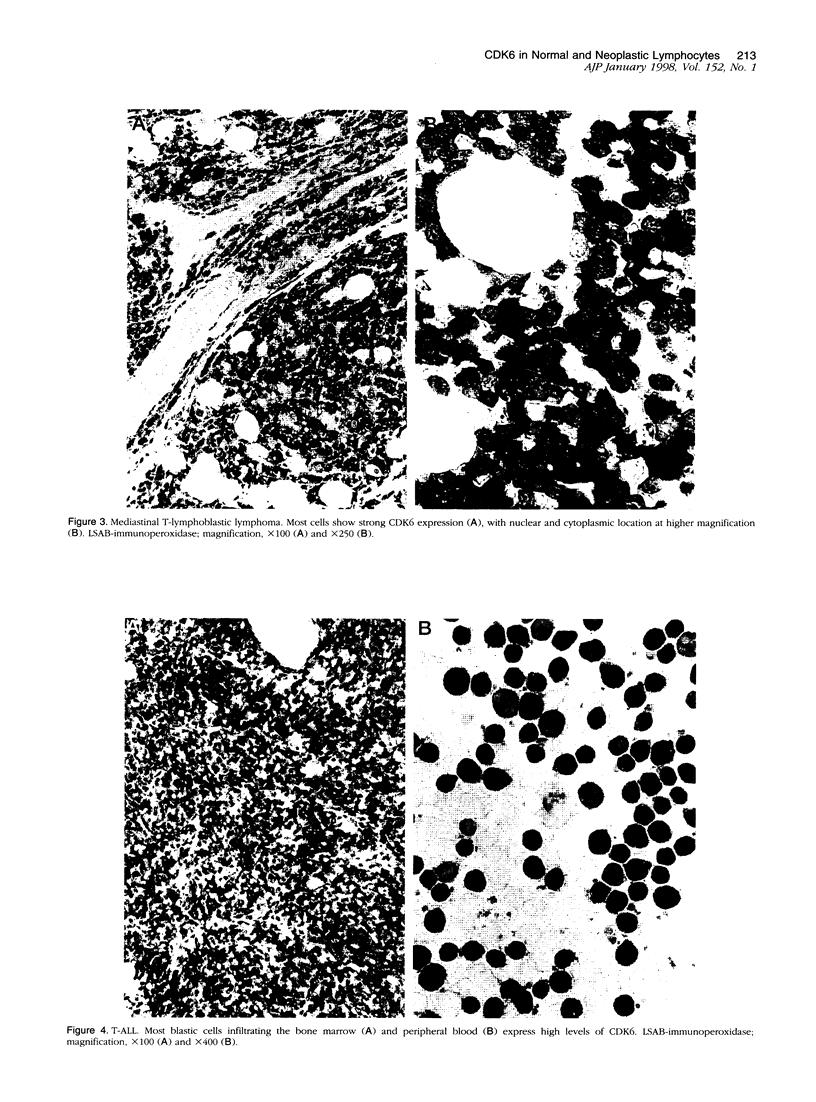
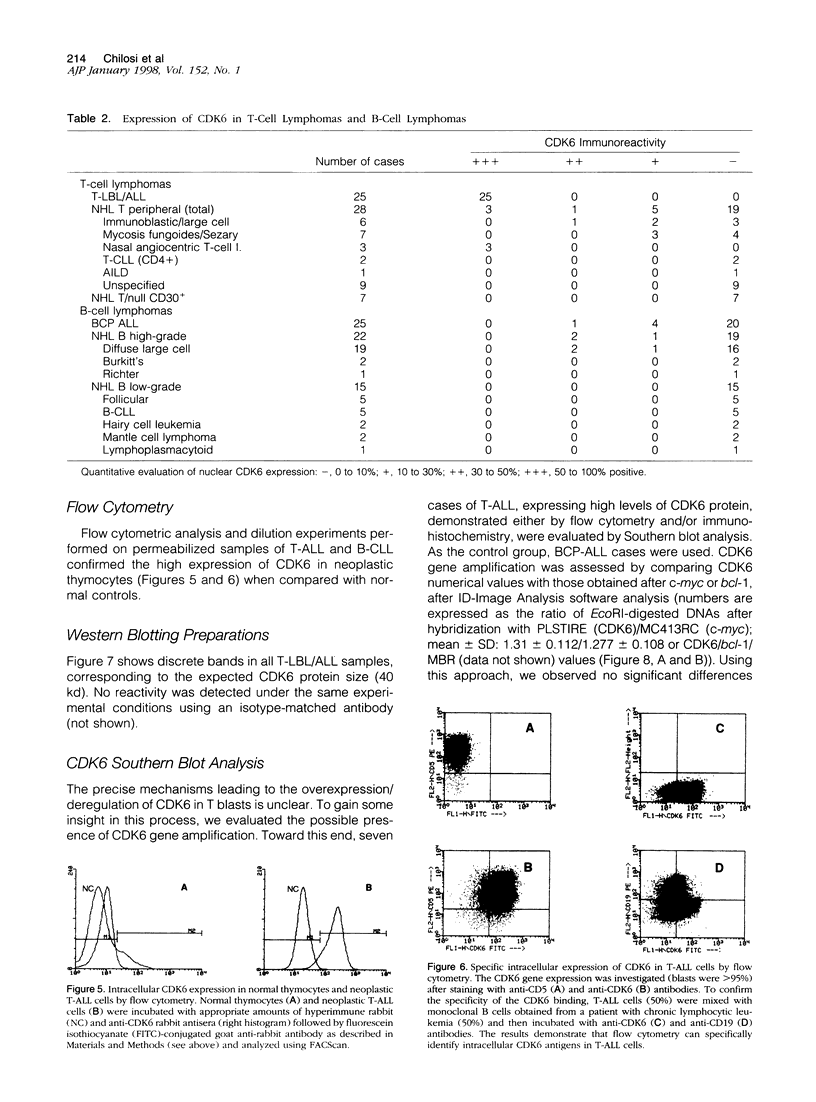
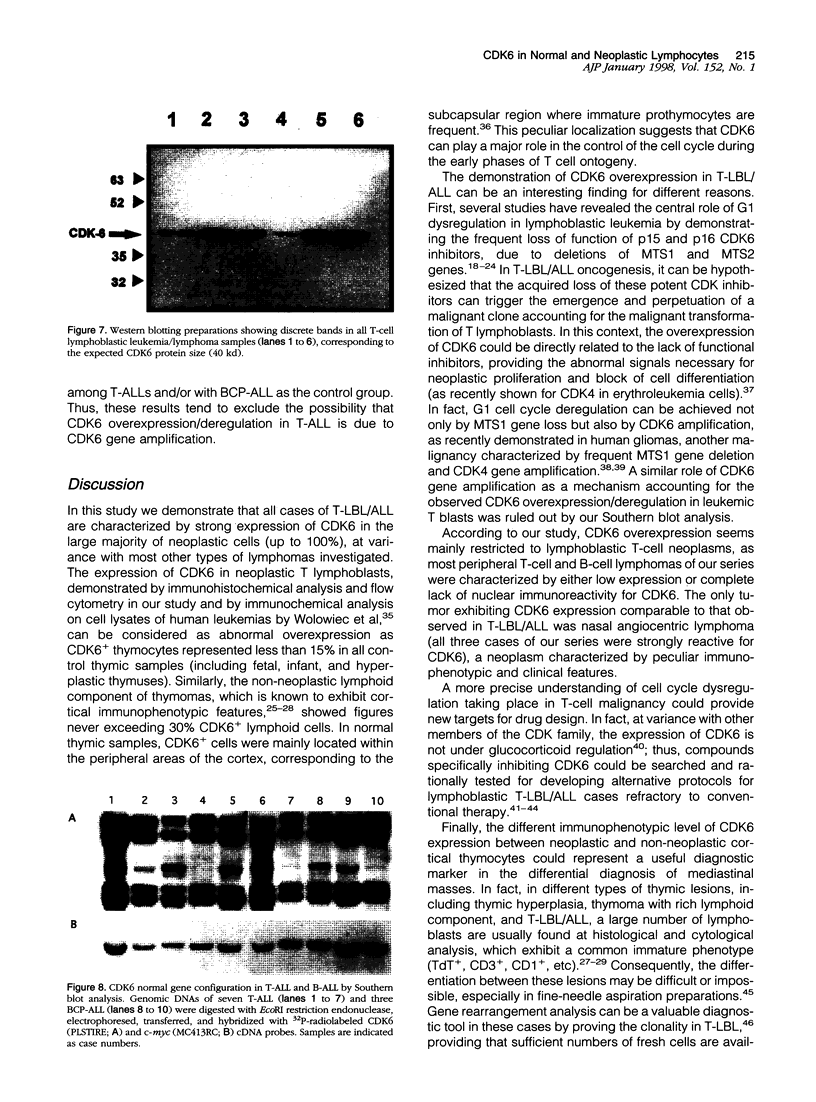
Abstract
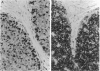
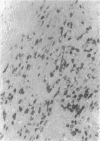
Cyclin-dependent kinase-6 (CDK6) is the earliest inducible member of the CDK family in human T lymphocytes, involved in growth factor stimulation and cell cycle progression. CDK6 is one of the targets of p16 and p15, CDK inhibitors encoded by MTS1 and MTS2, two tumor suppressor genes that are frequently deleted in T-cell leukemia. In this study we have investigated CDK6 expression in normal and neoplastic lymphoid tissues using immunohistochemistry and flow cytometry. In normal (six samples) and hyperplastic (four samples) thymuses, strong CDK6 expression was observed in a discrete proportion of cortical thymocytes (10 to 15%), mainly located in the peripheral (subcapsular) zone of the cortex. All tested cases of T-cell lymphoblastic lymphoma/leukemia (T-LBL/ALL) showed strong CDK6 expression in the majority (up to 100%) of neoplastic lymphoid cells. Western blot analysis confirmed the expected CDK6 protein size (40 kd). According to Southern blot analysis, CDK6 overexpression in neoplastic T lymphoblasts was not due to gene amplification. In all other lymphomas investigated (28 peripheral T-cell non-Hodgkin's lympohomas (T-NHLs), 7 CD30+ anaplastic NHLs, 22 high-grade B-NHLs, 15 low-grade B-NHLs, 25 B-cell precursor ALLs), CDK6 was not expressed or expressed at low levels, with the only exception of three nasal angiocentric T-NHLs, all exhibiting CDK6 immunoreactivity comparable to that observed in T-LBL/ALL. These data provide evidence that CDK6 is abnormally expressed in T-LBL/ALL and may be involved in the pathogenesis of this malignancy. In addition, the quantitative difference of CDK6 expression between neoplastic and non-neoplastic cortical thymocytes can be potentially useful in the differential diagnosis of thymic neoplasms on histological and cytological specimens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe J., Zhou W., Takuwa N., Taguchi J., Kurokawa K., Kumada M., Takuwa Y. A fumagillin derivative angiogenesis inhibitor, AGM-1470, inhibits activation of cyclin-dependent kinases and phosphorylation of retinoblastoma gene product but not protein tyrosyl phosphorylation or protooncogene expression in vascular endothelial cells. Cancer Res. 1994 Jul 1;54(13):3407–3412. [PubMed] [Google Scholar]
- Akagi T., Ono H., Shimotohno K. Tyrosine kinase inhibitor herbimycin A reduces the stability of cyclin-dependent kinase Cdk6 protein in T-cells. Oncogene. 1996 Jul 18;13(2):399–405. [PubMed] [Google Scholar]
- Bates S., Bonetta L., MacAllan D., Parry D., Holder A., Dickson C., Peters G. CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene. 1994 Jan;9(1):71–79. [PubMed] [Google Scholar]
- Cayuela J. M., Madani A., Sanhes L., Stern M. H., Sigaux F. Multiple tumor-suppressor gene 1 inactivation is the most frequent genetic alteration in T-cell acute lymphoblastic leukemia. Blood. 1996 Mar 15;87(6):2180–2186. [PubMed] [Google Scholar]
- Chilosi M., Doglioni C., Menestrina F., Montagna L., Rigo A., Lestani M., Barbareschi M., Scarpa A., Mariuzzi G. M., Pizzolo G. Abnormal expression of the p53-binding protein MDM2 in Hodgkin's disease. Blood. 1994 Dec 15;84(12):4295–4300. [PubMed] [Google Scholar]
- Chilosi M., Iannucci A. M., Pizzolo G., Menestrina F., Fiore-Donati L., Janossy G. Immunohistochemical analysis of thymoma. Evidence for medullary origin of epithelial cells. Am J Surg Pathol. 1984 Apr;8(4):309–318. doi: 10.1097/00000478-198404000-00009. [DOI] [PubMed] [Google Scholar]
- Chilosi M., Iannucci A., Fiore-Donati L., Tridente G., Pampanin M., Pizzolo G., Ritter M., Bofill M., Janossy G. Myasthenia gravis: immunohistological heterogeneity in microenvironmental organization of hyperplastic and neoplastic thymuses suggesting different mechanisms of tolerance breakdown. J Neuroimmunol. 1986 May;11(3):191–204. doi: 10.1016/0165-5728(86)90003-2. [DOI] [PubMed] [Google Scholar]
- Chilosi M., Iannucci A., Menestrina F., Lestani M., Scarpa A., Bonetti F., Fiore-Donati L., DiPasquale B., Pizzolo G., Palestro G. Immunohistochemical evidence of active thymocyte proliferation in thymoma. Its possible role in the pathogenesis of autoimmune diseases. Am J Pathol. 1987 Sep;128(3):464–470. [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol. 1995 Sep;147(3):545–560. [PMC free article] [PubMed] [Google Scholar]
- Friedman H. D., Hutchison R. E., Kohman L. J., Powers C. N. Thymoma mimicking lymphoblastic lymphoma: a pitfall in fine-needle aspiration biopsy interpretation. Diagn Cytopathol. 1996 Mar;14(2):165–171. doi: 10.1002/(SICI)1097-0339(199603)14:2<165::AID-DC12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Jenkins C. W., Li Y., Nichols M. A., Wu X., O'Keefe C. L., Matera A. G., Xiong Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994 Dec 15;8(24):2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- Hall M., Bates S., Peters G. Evidence for different modes of action of cyclin-dependent kinase inhibitors: p15 and p16 bind to kinases, p21 and p27 bind to cyclins. Oncogene. 1995 Oct 19;11(8):1581–1588. [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Hatta Y., Hirama T., Miller C. W., Yamada Y., Tomonaga M., Koeffler H. P. Homozygous deletions of the p15 (MTS2) and p16 (CDKN2/MTS1) genes in adult T-cell leukemia. Blood. 1995 May 15;85(10):2699–2704. [PubMed] [Google Scholar]
- Hebert J., Cayuela J. M., Berkeley J., Sigaux F. Candidate tumor-suppressor genes MTS1 (p16INK4A) and MTS2 (p15INK4B) display frequent homozygous deletions in primary cells from T- but not from B-cell lineage acute lymphoblastic leukemias. Blood. 1994 Dec 15;84(12):4038–4044. [PubMed] [Google Scholar]
- Hirama T., Koeffler H. P. Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood. 1995 Aug 1;86(3):841–854. [PubMed] [Google Scholar]
- Inghirami G., Foitl D. R., Sabichi A., Zhu B. Y., Knowles D. M. Autoantibody-associated cross-reactive idiotype-bearing human B lymphocytes: distribution and characterization, including Ig VH gene and CD5 antigen expression. Blood. 1991 Sep 15;78(6):1503–1515. [PubMed] [Google Scholar]
- Janossy G., Bofill M., Trejdosiewicz L. K., Willcox H. N., Chilosi M. Cellular differentiation of lymphoid subpopulations and their microenvironments in the human thymus. Curr Top Pathol. 1986;75:89–125. doi: 10.1007/978-3-642-82480-7_3. [DOI] [PubMed] [Google Scholar]
- Karp J. E., Broder S. Molecular foundations of cancer: new targets for intervention. Nat Med. 1995 Apr;1(4):309–320. doi: 10.1038/nm0495-309. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H., Richon V. M., Rifkind R. A., Marks P. A. Suppression of cyclin-dependent kinase 4 during induced differentiation of erythroleukemia cells. Mol Cell Biol. 1994 Nov;14(11):7195–7203. doi: 10.1128/mcb.14.11.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T. K., Buchholz M. A., Gabrielson E. W., Nordin A. A. A novel cytoplasmic substrate for cdk4 and cdk6 in normal and malignant epithelial derived cells. Oncogene. 1995 Nov 16;11(10):2077–2083. [PubMed] [Google Scholar]
- Lucas J. J., Szepesi A., Modiano J. F., Domenico J., Gelfand E. W. Regulation of synthesis and activity of the PLSTIRE protein (cyclin-dependent kinase 6 (cdk6)), a major cyclin D-associated cdk4 homologue in normal human T lymphocytes. J Immunol. 1995 Jun 15;154(12):6275–6284. [PubMed] [Google Scholar]
- Marino M., Müller-Hermelink H. K. Thymoma and thymic carcinoma. Relation of thymoma epithelial cells to the cortical and medullary differentiation of thymus. Virchows Arch A Pathol Anat Histopathol. 1985;407(2):119–149. doi: 10.1007/BF00737071. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Richon V. M., Kiyokawa H., Rifkind R. A. Inducing differentiation of transformed cells with hybrid polar compounds: a cell cycle-dependent process. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10251–10254. doi: 10.1073/pnas.91.22.10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H., Quelle D. E., Shurtleff S. A., Shibuya M., Sherr C. J., Kato J. Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994 Mar;14(3):2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson M., Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994 Mar;14(3):2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modiano J. F., Domenico J., Szepesi A., Lucas J. J., Gelfand E. W. Differential requirements for interleukin-2 distinguish the expression and activity of the cyclin-dependent kinases Cdk4 and Cdk2 in human T cells. J Biol Chem. 1994 Dec 30;269(52):32972–32978. [PubMed] [Google Scholar]
- Ohnishi H., Kawamura M., Ida K., Sheng X. M., Hanada R., Nobori T., Yamamori S., Hayashi Y. Homozygous deletions of p16/MTS1 gene are frequent but mutations are infrequent in childhood T-cell acute lymphoblastic leukemia. Blood. 1995 Aug 15;86(4):1269–1275. [PubMed] [Google Scholar]
- Okuda T., Shurtleff S. A., Valentine M. B., Raimondi S. C., Head D. R., Behm F., Curcio-Brint A. M., Liu Q., Pui C. H., Sherr C. J. Frequent deletion of p16INK4a/MTS1 and p15INK4b/MTS2 in pediatric acute lymphoblastic leukemia. Blood. 1995 May 1;85(9):2321–2330. [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Magrath I., Dalla-Favera R. Chromosomal breakpoints and structural alterations of the c-myc locus differ in endemic and sporadic forms of Burkitt lymphoma. Proc Natl Acad Sci U S A. 1986 May;83(9):2984–2988. doi: 10.1073/pnas.83.9.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel B., Preudhomme C., Philippe N., Vanrumbeke M., Dervite I., Lai J. L., Bauters F., Wattel E., Fenaux P. p16 gene homozygous deletions in acute lymphoblastic leukemia. Blood. 1995 Feb 1;85(3):657–663. [PubMed] [Google Scholar]
- Rhee K., Reisman D., Bresnahan W., Thompson E. A. Glucocorticoid regulation of G1 cyclin-dependent kinase genes in lymphoid cells. Cell Growth Differ. 1995 Jun;6(6):691–698. [PubMed] [Google Scholar]
- Scarpa A., Chilosi M., Capelli P., Bonetti F., Menestrina F., Zamboni G., Pizzolo G., Palestro G., Fiore Donati L., Tridente G. Expression and gene rearrangement of the T-cell receptor in human thymomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;58(3):235–239. doi: 10.1007/BF02890077. [DOI] [PubMed] [Google Scholar]
- Schmidt E. E., Ichimura K., Reifenberger G., Collins V. P. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 1994 Dec 15;54(24):6321–6324. [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Strauss M., Lukas J., Bartek J. Unrestricted cell cycling and cancer. Nat Med. 1995 Dec;1(12):1245–1246. doi: 10.1038/nm1295-1245. [DOI] [PubMed] [Google Scholar]
- Szepesi A., Gelfand E. W., Lucas J. J. Association of proliferating cell nuclear antigen with cyclin-dependent kinases and cyclins in normal and transformed human T lymphocytes. Blood. 1994 Nov 15;84(10):3413–3421. [PubMed] [Google Scholar]
- Takeuchi S., Bartram C. R., Seriu T., Miller C. W., Tobler A., Janssen J. W., Reiter A., Ludwig W. D., Zimmermann M., Schwaller J. Analysis of a family of cyclin-dependent kinase inhibitors: p15/MTS2/INK4B, p16/MTS1/INK4A, and p18 genes in acute lymphoblastic leukemia of childhood. Blood. 1995 Jul 15;86(2):755–760. [PubMed] [Google Scholar]
- Tam S. W., Theodoras A. M., Shay J. W., Draetta G. F., Pagano M. Differential expression and regulation of Cyclin D1 protein in normal and tumor human cells: association with Cdk4 is required for Cyclin D1 function in G1 progression. Oncogene. 1994 Sep;9(9):2663–2674. [PubMed] [Google Scholar]
- Tsujimoto Y., Jaffe E., Cossman J., Gorham J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985 May 23;315(6017):340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Veselý J., Havlicek L., Strnad M., Blow J. J., Donella-Deana A., Pinna L., Letham D. S., Kato J., Detivaud L., Leclerc S. Inhibition of cyclin-dependent kinases by purine analogues. Eur J Biochem. 1994 Sep 1;224(2):771–786. doi: 10.1111/j.1432-1033.1994.00771.x. [DOI] [PubMed] [Google Scholar]
- Wołowiec D., Mekki Y., Ffrench P., Manel A. M., Bertrand Y., Rimokh R., Philippe N., Bryon P. A., Ffrench M. Differential expression of cell proliferation regulatory proteins in B- and T-lineage acute lymphoblastic leukaemias. Br J Haematol. 1996 Dec;95(3):518–523. doi: 10.1046/j.1365-2141.1996.d01-1930.x. [DOI] [PubMed] [Google Scholar]