Abstract
Inhibitors of programmed cell death (apoptosis) may regulate tissue differentiation and aberrantly promote cell survival in neoplasia. A novel apoptosis inhibitor of the IAP gene family, designated survivin, was recently found in all of the most common human cancers but not in normal, terminally differentiated adult tissues. The expression of survivin in embryonic and fetal development was investigated. Immunohistochemistry and in situ hybridization studies demonstrated strong expression of survivin in several apoptosis-regulated fetal tissues, including the stem cell layer of stratified epithelia, endocrine pancreas, and thymic medulla, with a pattern that did not overlap with that of another apoptosis inhibitor, bcl-2. A sequence-specific antibody to survivin immunoblotted a single approximately 16.5-kd survivin band in human fetal lung, liver, heart, kidney, and gastrointestinal tract. In mouse embryo, prominent and nearly ubiquitous distribution of survivin was found at embryonic day (E)11.5, whereas at E15 to -21, survivin expression was restricted to the distal bronchiolar epithelium of the lung and neural-crest-derived cells, including dorsal root ganglion neurons, hypophysis, and the choroid plexus. These data suggest that expression of survivin in embryonic and fetal development may contribute to tissue homeostasis and differentiation independently of bcl-2. Aberrations of this developmental pathway may result in prominent re-expression of survivin in neoplasia and abnormally prolonged cell viability.
Full text
PDF


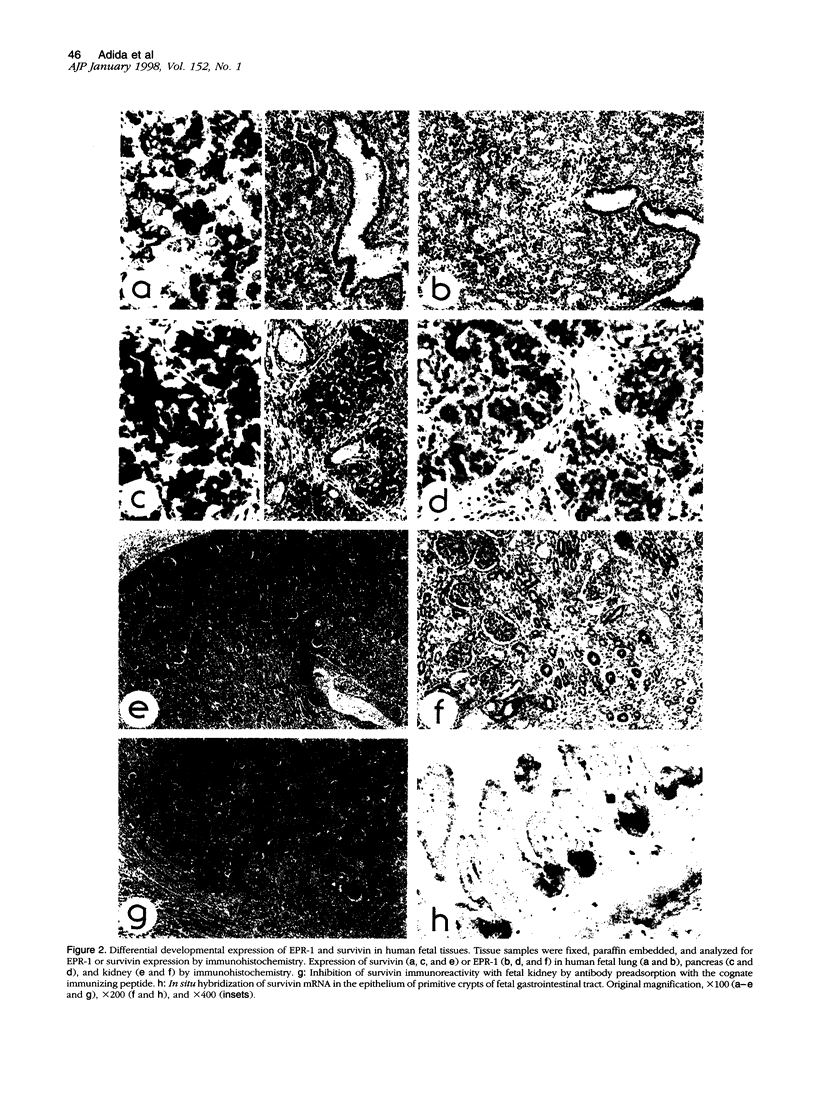
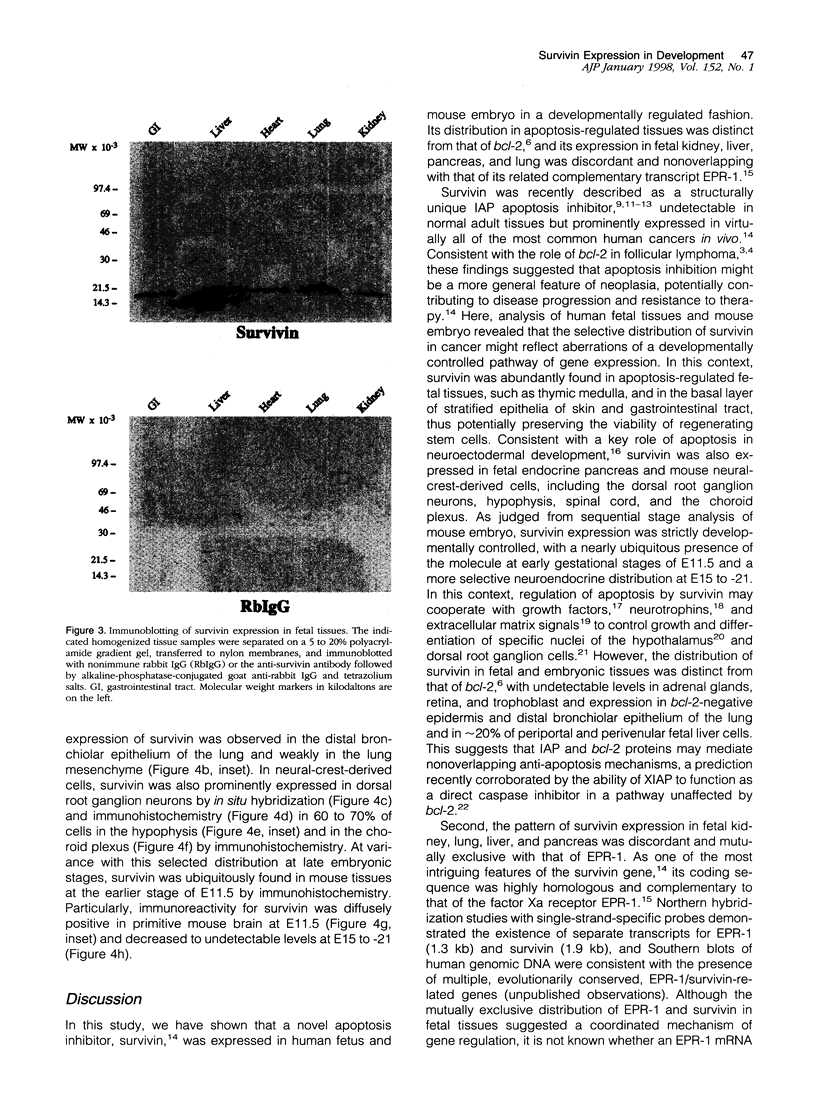

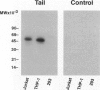
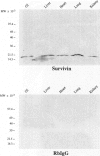
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri D. C. Xa receptor EPR-1. FASEB J. 1995 Jul;9(10):860–865. doi: 10.1096/fasebj.9.10.7615156. [DOI] [PubMed] [Google Scholar]
- Ambrosini G., Adida C., Altieri D. C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997 Aug;3(8):917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Celano P., Berchtold C. M., Kizer D. L., Weeraratna A., Nelkin B. D., Baylin S. B., Casero R. A., Jr Characterization of an endogenous RNA transcript with homology to the antisense strand of the human c-myc gene. J Biol Chem. 1992 Jul 25;267(21):15092–15096. [PubMed] [Google Scholar]
- Clem R. J., Miller L. K. Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol. 1994 Aug;14(8):5212–5222. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R. E., Pover C. M., Fitzgerald M. Dorsal root ganglion cell death and surviving cell numbers in relation to the development of sensory innervation in the rat hindlimb. Brain Res Dev Brain Res. 1994 Oct 14;82(1-2):193–212. doi: 10.1016/0165-3806(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Davies A. M. Neurotrophic factors. Switching neurotrophin dependence. Curr Biol. 1994 Mar 1;4(3):273–276. doi: 10.1016/s0960-9822(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Davis E. C., Popper P., Gorski R. A. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996 Sep 23;734(1-2):10–18. [PubMed] [Google Scholar]
- Deveraux Q. L., Takahashi R., Salvesen G. S., Reed J. C. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997 Jul 17;388(6639):300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- Duckett C. S., Nava V. E., Gedrich R. W., Clem R. J., Van Dongen J. L., Gilfillan M. C., Shiels H., Hardwick J. M., Thompson C. B. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 1996 Jun 3;15(11):2685–2694. [PMC free article] [PubMed] [Google Scholar]
- Hay B. A., Wassarman D. A., Rubin G. M. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell. 1995 Dec 29;83(7):1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung W., Castren E., Odenthal M., Vande Woude G. F., Ishii T., Dienes H. P., Lindholm D., Schirmacher P. Expression and functional interaction of hepatocyte growth factor-scatter factor and its receptor c-met in mammalian brain. J Cell Biol. 1994 Jul;126(2):485–494. doi: 10.1083/jcb.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khochbin S., Lawrence J. J. An antisense RNA involved in p53 mRNA maturation in murine erythroleukemia cells induced to differentiate. EMBO J. 1989 Dec 20;8(13):4107–4114. doi: 10.1002/j.1460-2075.1989.tb08595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. W. An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell. 1989 Nov 17;59(4):687–696. doi: 10.1016/0092-8674(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Krystal G. W., Armstrong B. C., Battey J. F. N-myc mRNA forms an RNA-RNA duplex with endogenous antisense transcripts. Mol Cell Biol. 1990 Aug;10(8):4180–4191. doi: 10.1128/mcb.10.8.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrun D. P., Warnke R. A., Cleary M. L. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol. 1993 Mar;142(3):743–753. [PMC free article] [PubMed] [Google Scholar]
- Liston P., Roy N., Tamai K., Lefebvre C., Baird S., Cherton-Horvat G., Farahani R., McLean M., Ikeda J. E., MacKenzie A. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996 Jan 25;379(6563):349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- Oltvai Z. N., Korsmeyer S. J. Checkpoints of dueling dimers foil death wishes. Cell. 1994 Oct 21;79(2):189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M., Pan M. G., Henzel W. J., Ayres T. M., Goeddel D. V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995 Dec 29;83(7):1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Roy N., Mahadevan M. S., McLean M., Shutler G., Yaraghi Z., Farahani R., Baird S., Besner-Johnston A., Lefebvre C., Kang X. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995 Jan 13;80(1):167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- Uren A. G., Pakusch M., Hawkins C. J., Puls K. L., Vaux D. L. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. L., Haecker G., Strasser A. An evolutionary perspective on apoptosis. Cell. 1994 Mar 11;76(5):777–779. doi: 10.1016/0092-8674(94)90350-6. [DOI] [PubMed] [Google Scholar]
- Yang E., Korsmeyer S. J. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996 Jul 15;88(2):386–401. [PubMed] [Google Scholar]
- Zhang Z., Vuori K., Reed J. C., Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]