Abstract
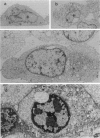
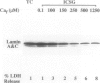
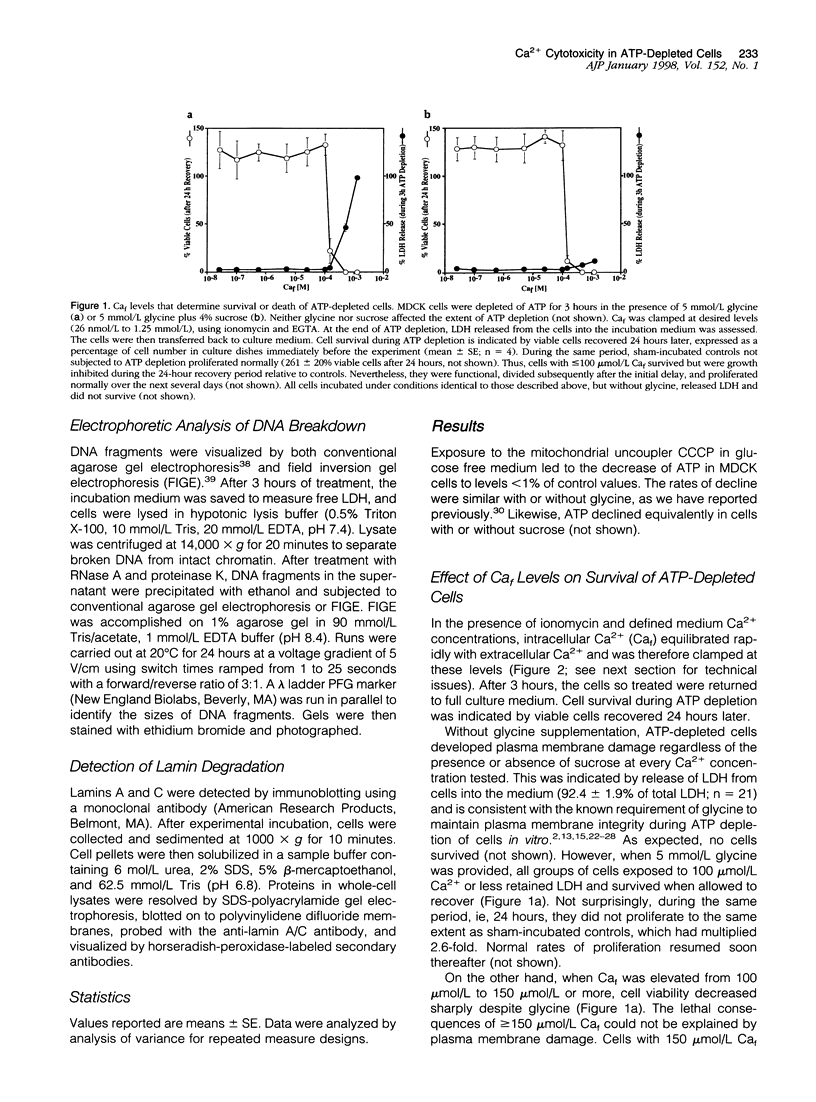
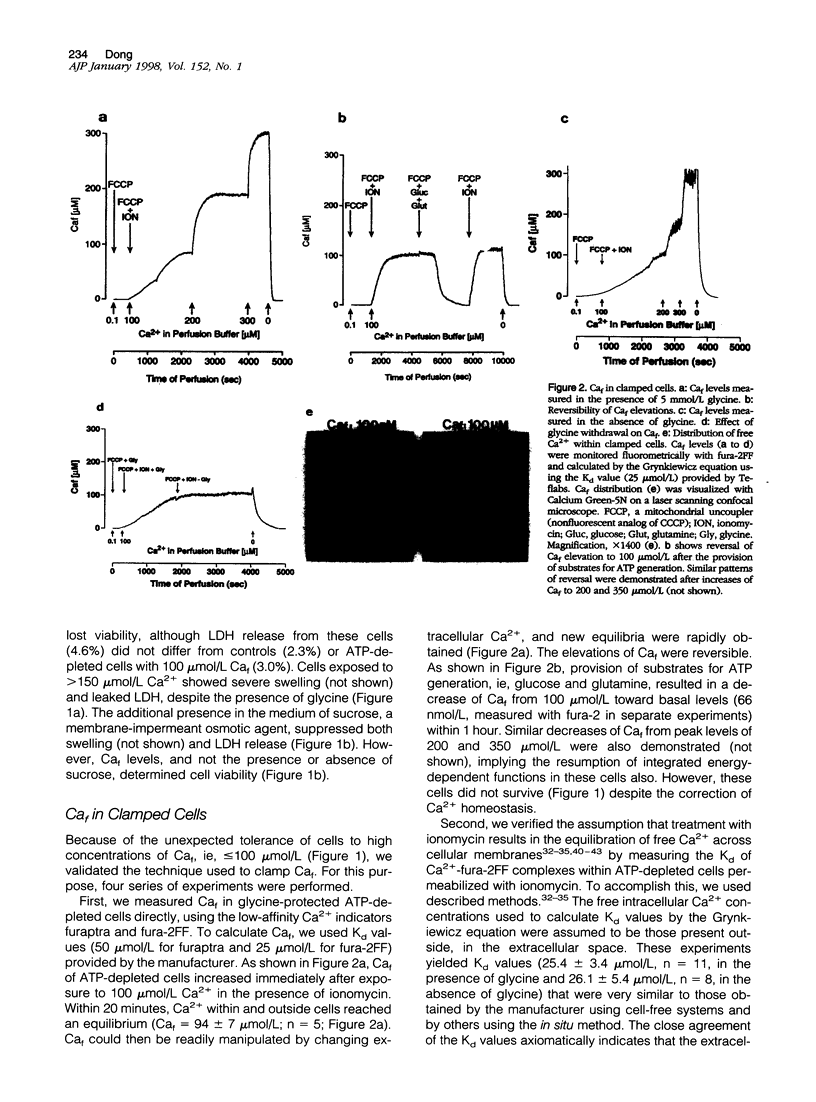
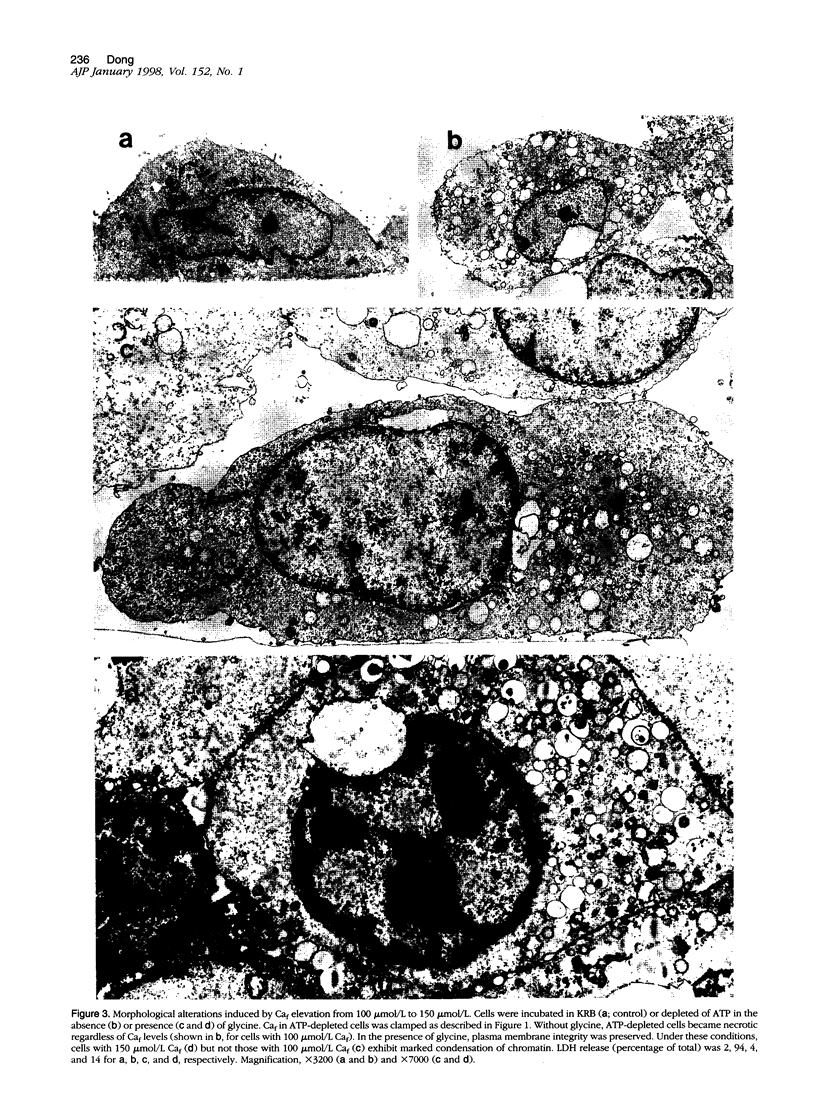
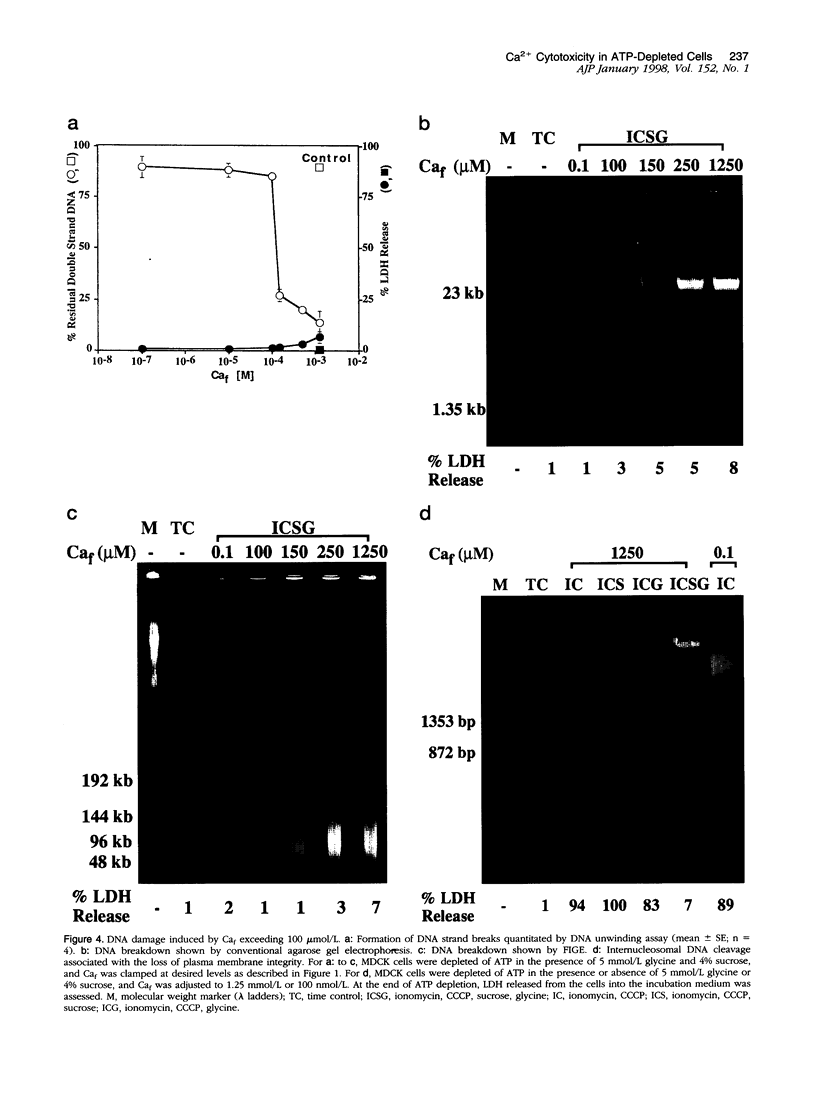
Increase of intracellular ionized or free Ca2+ is thought to play a central role in cell death due to ATP depletion. However, concurrently operative mechanisms of injury that do not require intracellular Ca2+ increases have made it difficult to test this hypothesis or to determine the concentrations at which intracellular Ca2+ becomes lethal. The predominant Ca2+-independent mechanism of injury during ATP depletion involves the loss of cellular glycine. This type of damage can be fully inhibited by adding the amino acid exogenously. Using glycine to suppress Ca2+-independent plasma membrane damage, we have examined the effect of intracellular Ca2+ elevations on cell viability during ATP depletion. Madin-Darby canine kidney (MDCK) cells were depleted of ATP by incubation with a mitochondrial uncoupler in glucose-free medium. Free Ca2+ concentration in the medium was varied between 26 nmol/L and 1.25 mmol/L in the presence of a Ca2+ ionophore. Measurements with the Ca2+ probes fura-2, furaptra, and fura-2FF showed that intracellular Ca2+ was clamped at extracellular levels under these conditions. Cell survival during ATP depletion was indicated by viable cells recovered 24 hours later. The results show that ATP-depleted cells can sustain high levels of intracellular Ca2+ (100 micromol/L) for prolonged periods and remain viable if plasma membrane damage is prevented by glycine. Cell death was observed only when intracellular free Ca2+ was allowed to increase beyond 100 micromol/L, and this was associated with dramatic nuclear alterations: chromatin condensation, loss of nuclear lamins, and breakdown of DNA into large 50- to 150-kb fragments. Our studies demonstrate unexpectedly high resistance of cells to calcium cytotoxicity if glycine that is lost during ATP depletion is restored. In addition, they provide insights into novel mechanisms of nuclear disintegration and DNA damage that are triggered when the high thresholds of intracellular Ca2+ required for cell death are exceeded.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Beck F. X., Ohno A., Dörge A., Thurau K. Ischemia-induced changes in cell element composition and osmolyte contents of outer medulla. Kidney Int. 1995 Aug;48(2):449–457. doi: 10.1038/ki.1995.313. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. The biology and medicine of calcium signalling. Mol Cell Endocrinol. 1994 Jan;98(2):119–124. doi: 10.1016/0303-7207(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. Fluorometric analysis of DNA unwinding to study strand breaks and repair in mammalian cells. Methods Enzymol. 1990;186:550–555. doi: 10.1016/0076-6879(90)86149-p. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V. Mediators of ischemic renal injury. Annu Rev Med. 1988;39:531–544. doi: 10.1146/annurev.me.39.020188.002531. [DOI] [PubMed] [Google Scholar]
- Boyles J., Fox J. E., Phillips D. R., Stenberg P. E. Organization of the cytoskeleton in resting, discoid platelets: preservation of actin filaments by a modified fixation that prevents osmium damage. J Cell Biol. 1985 Oct;101(4):1463–1472. doi: 10.1083/jcb.101.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
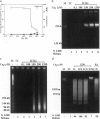
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Chi W. M., Berezesky I. K., Smith M. W., Trump B. F. Changes in [Ca2+]i in cultured rat proximal tubular epithelium: an in vitro model for renal ischemia. Biochim Biophys Acta. 1995 Apr 13;1243(3):513–520. doi: 10.1016/0304-4165(94)00190-9. [DOI] [PubMed] [Google Scholar]
- Currin R. T., Caldwell-Kenkel J. C., Lichtman S. N., Bachmann S., Takei Y., Kawano S., Thurman R. G., Lemasters J. J. Protection by Carolina rinse solution, acidotic pH, and glycine against lethal reperfusion injury to sinusoidal endothelial cells of rat livers stored for transplantation. Transplantation. 1996 Dec 15;62(11):1549–1558. doi: 10.1097/00007890-199612150-00004. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Bronk S. F., Gores G. J. Glycine cytoprotection during lethal hepatocellular injury from adenosine triphosphate depletion. Gastroenterology. 1992 Jun;102(6):2098–2107. doi: 10.1016/0016-5085(92)90338-y. [DOI] [PubMed] [Google Scholar]
- Dong Z., Saikumar P., Weinberg J. M., Venkatachalam M. A. Internucleosomal DNA cleavage triggered by plasma membrane damage during necrotic cell death. Involvement of serine but not cysteine proteases. Am J Pathol. 1997 Nov;151(5):1205–1213. [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Chien K. R., Mittnacht S., Jr Myocardial ischemia: the pathogenesis of irreversible cell injury in ischemia. Am J Pathol. 1981 Feb;102(2):271–281. [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A., Frey M., Fleckenstein-Grün G. Consequences of uncontrolled calcium entry and its prevention with calcium antagonists. Eur Heart J. 1983 Dec;4 (Suppl H):43–50. doi: 10.1093/eurheartj/4.suppl_h.43. [DOI] [PubMed] [Google Scholar]
- Gasbarrini A., Borle A. B., Farghali H., Bender C., Francavilla A., Van Thiel D. Effect of anoxia on intracellular ATP, Na+i, Ca2+i, Mg2+i, and cytotoxicity in rat hepatocytes. J Biol Chem. 1992 Apr 5;267(10):6654–6663. [PubMed] [Google Scholar]
- Golovina V. A., Blaustein M. P. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997 Mar 14;275(5306):1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hansen A. J. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985 Jan;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Hansen C. A., Monck J. R., Williamson J. R. Measurement of intracellular free calcium to investigate receptor-mediated calcium signaling. Methods Enzymol. 1990;191:691–706. doi: 10.1016/0076-6879(90)91042-5. [DOI] [PubMed] [Google Scholar]
- Herman B., Gores G. J., Nieminen A. L., Kawanishi T., Harman A., Lemasters J. J. Calcium and pH in anoxic and toxic injury. Crit Rev Toxicol. 1990;21(2):127–148. doi: 10.3109/10408449009089876. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W. Defense strategies against hypoxia and hypothermia. Science. 1986 Jan 17;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hofer A. M., Schlue W. R., Curci S., Machen T. E. Spatial distribution and quantitation of free luminal [Ca] within the InsP3-sensitive internal store of individual BHK-21 cells: ion dependence of InsP3-induced Ca release and reloading. FASEB J. 1995 Jun;9(9):788–798. doi: 10.1096/fasebj.9.9.7601343. [DOI] [PubMed] [Google Scholar]
- Hurley T. W., Ryan M. P., Brinck R. W. Changes of cytosolic Ca2+ interfere with measurements of cytosolic Mg2+ using mag-fura-2. Am J Physiol. 1992 Aug;263(2 Pt 1):C300–C307. doi: 10.1152/ajpcell.1992.263.2.C300. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A. The cell biology of acute myocardial ischemia. Annu Rev Med. 1991;42:225–246. doi: 10.1146/annurev.me.42.020191.001301. [DOI] [PubMed] [Google Scholar]
- Kribben A., Wieder E. D., Wetzels J. F., Yu L., Gengaro P. E., Burke T. J., Schrier R. W. Evidence for role of cytosolic free calcium in hypoxia-induced proximal tubule injury. J Clin Invest. 1994 May;93(5):1922–1929. doi: 10.1172/JCI117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik Y. A., Takahashi A., Moir R. D., Goldman R. D., Poirier G. G., Kaufmann S. H., Earnshaw W. C. Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9042–9046. doi: 10.1073/pnas.92.20.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L., García-Sancho J. Measurement and control of intracellular calcium in intact red cells. Methods Enzymol. 1989;173:100–112. doi: 10.1016/s0076-6879(89)73008-1. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Schnellmann R. G., Jacobs W. R. Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest. 1990 Feb;85(2):316–324. doi: 10.1172/JCI114440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino J. E., Kotadia B., Mangino M. J. Characterization of hypothermic intestinal ischemia-reperfusion injury in dogs. Effects of glycine. Transplantation. 1996 Jul 27;62(2):173–178. doi: 10.1097/00007890-199607270-00005. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Halestrap A. P., Denton R. M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990 Apr;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- McCoy C. E., Selvaggio A. M., Alexander E. A., Schwartz J. H. Adenosine triphosphate depletion induces a rise in cytosolic free calcium in canine renal epithelial cells. J Clin Invest. 1988 Oct;82(4):1326–1332. doi: 10.1172/JCI113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Thor H., Orrenius S. Cytosolic-free Ca2+ and cell killing in hepatoma 1c1c7 cells exposed to chemical anoxia. FASEB J. 1989 Jan;3(1):59–64. doi: 10.1096/fasebj.3.1.2910738. [DOI] [PubMed] [Google Scholar]
- Rao L., Perez D., White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996 Dec;135(6 Pt 1):1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Rasmussen J. E. Calcium as intracellular messenger: from simplicity to complexity. Curr Top Cell Regul. 1990;31:1–109. doi: 10.1016/b978-0-12-152831-7.50003-2. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Historical overview. Calcium, ischemia, and death of brain cells. Ann N Y Acad Sci. 1988;522:638–661. doi: 10.1111/j.1749-6632.1988.tb33410.x. [DOI] [PubMed] [Google Scholar]
- Silva P., Rosen S., Spokes K., Epstein F. H. Effect of glycine on medullary thick ascending limb injury in perfused kidneys. Kidney Int. 1991 Apr;39(4):653–658. doi: 10.1038/ki.1991.78. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Goldman W. F. Measurement of SR free Ca2+ and Mg2+ in permeabilized smooth muscle cells with use of furaptra. Am J Physiol. 1995 Sep;269(3 Pt 1):C698–C705. doi: 10.1152/ajpcell.1995.269.3.C698. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K. Calcium-mediated cell injury and cell death. FASEB J. 1995 Feb;9(2):219–228. doi: 10.1096/fasebj.9.2.7781924. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Weinberg J. M., Patel Y., Saikumar P., Dong Z. Cytoprotection of kidney epithelial cells by compounds that target amino acid gated chloride channels. Kidney Int. 1996 Feb;49(2):449–460. doi: 10.1038/ki.1996.64. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Roeser N. F., Venkatachalam M. A. Role of increased cytosolic free calcium in the pathogenesis of rabbit proximal tubule cell injury and protection by glycine or acidosis. J Clin Invest. 1991 Feb;87(2):581–590. doi: 10.1172/JCI115033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Venkatachalam M. A. Cytosolic-free calcium increases to greater than 100 micromolar in ATP-depleted proximal tubules. J Clin Invest. 1997 Aug 1;100(3):713–722. doi: 10.1172/JCI119584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M. The cell biology of ischemic renal injury. Kidney Int. 1991 Mar;39(3):476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Venkatachalam M. A., Garzo-Quintero R., Roeser N. F., Davis J. A. Structural requirements for protection by small amino acids against hypoxic injury in kidney proximal tubules. FASEB J. 1990 Dec;4(15):3347–3354. doi: 10.1096/fasebj.4.15.2253849. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Venkatachalam M. A., Roeser N. F., Davis J. A., Varani J., Johnson K. J. Amino acid protection of cultured kidney tubule cells against calcium ionophore-induced lethal cell injury. Lab Invest. 1991 Dec;65(6):671–678. [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Calcium. 1990 Feb-Mar;11(2-3):75–83. doi: 10.1016/0143-4160(90)90061-x. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Jones S., Thurman R. G. Glycine minimizes reperfusion injury in a low-flow, reflow liver perfusion model in the rat. Am J Physiol. 1996 Feb;270(2 Pt 1):G332–G338. doi: 10.1152/ajpgi.1996.270.2.G332. [DOI] [PubMed] [Google Scholar]