Abstract
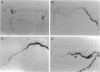
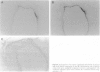
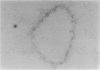
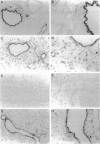
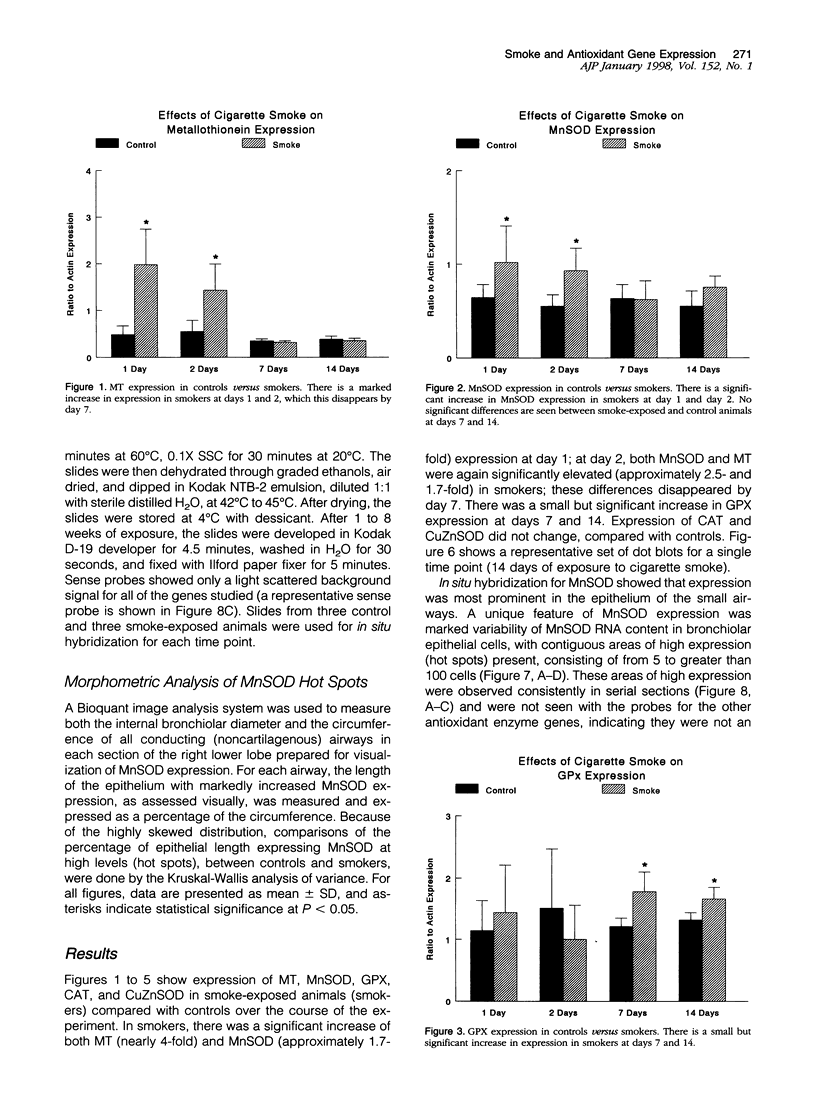
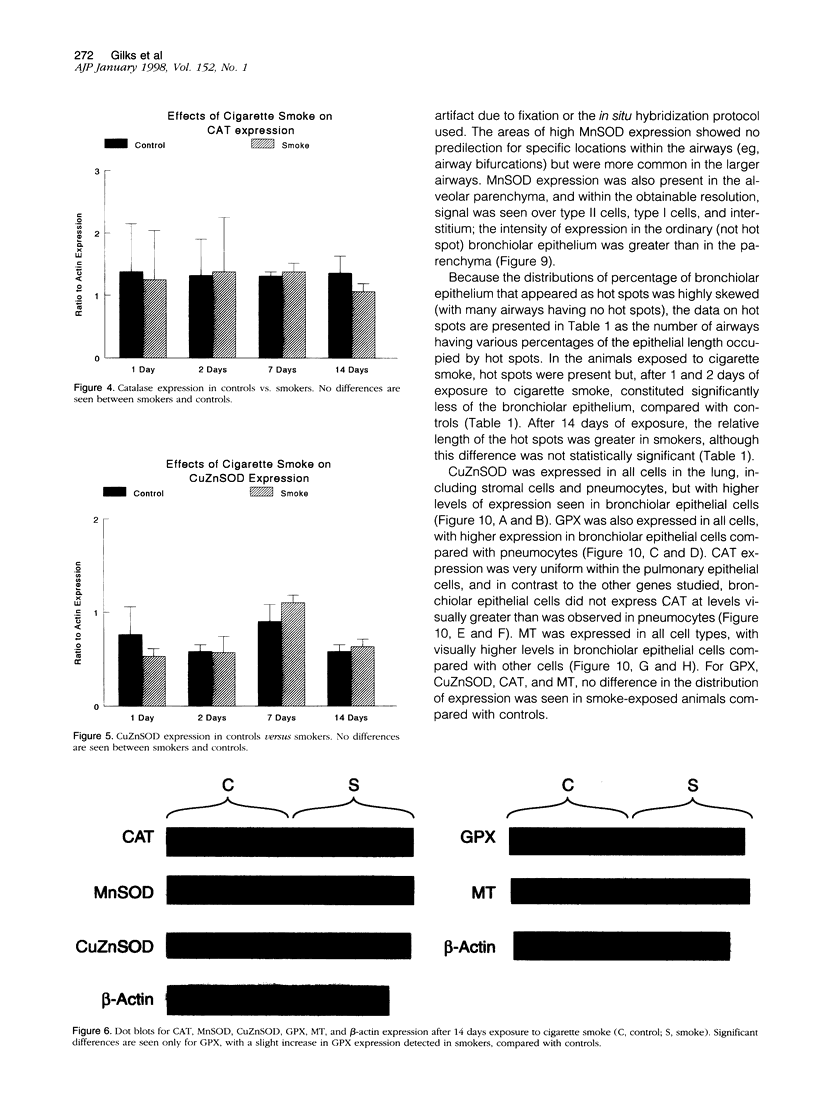
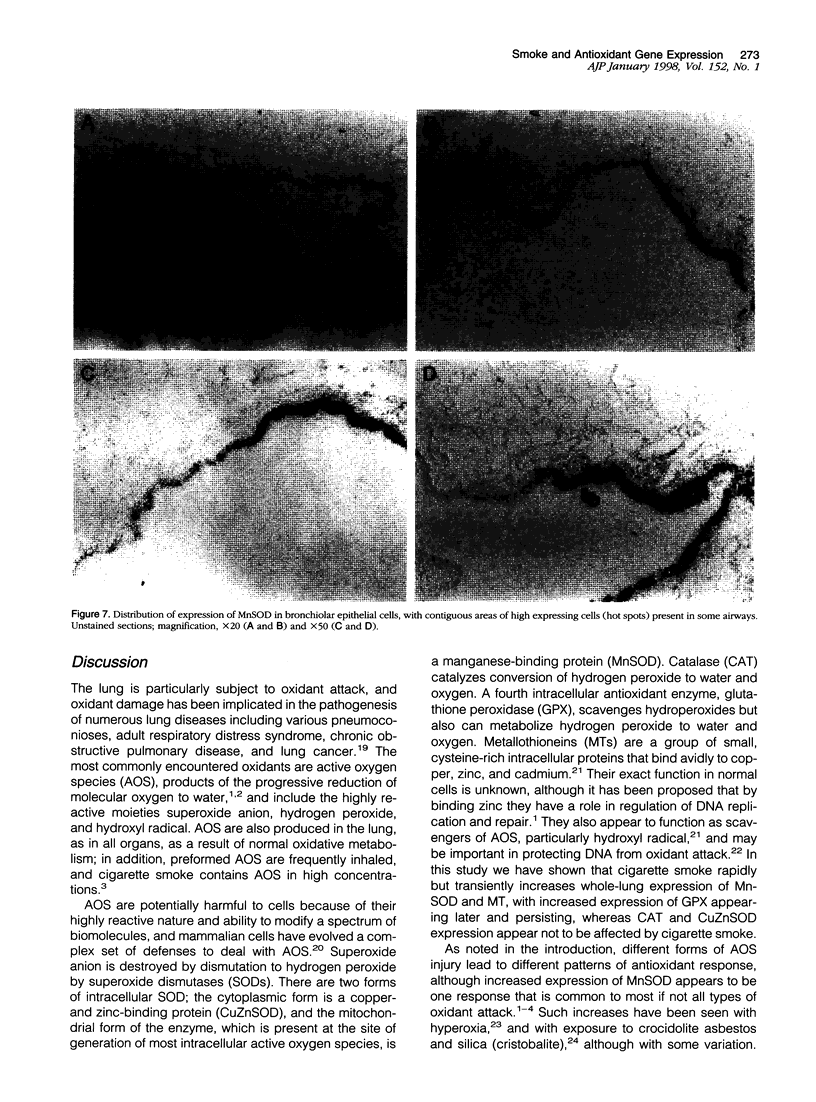
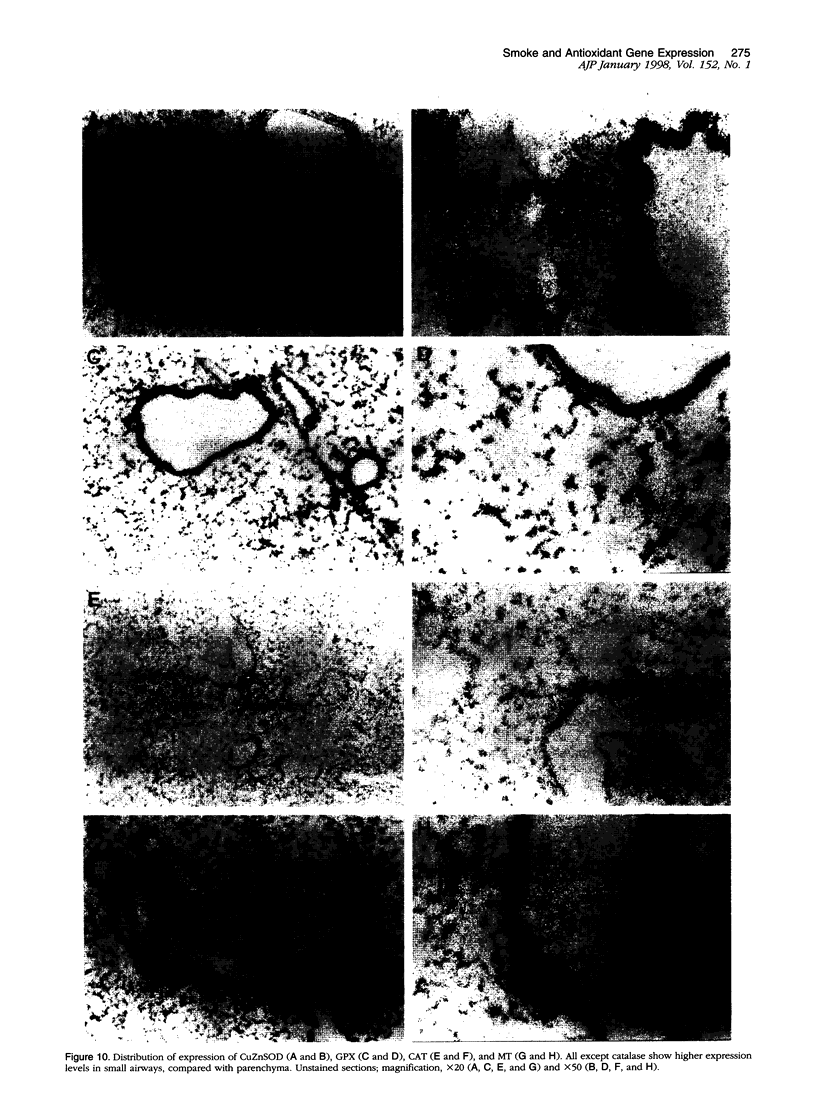
To investigate the effects of cigarette smoke on the expression of genes encoding intracellular antioxidant species, we exposed rats to whole cigarette smoke or air (control) daily for 1, 2, 7, or 14 days. After sacrifice, RNA was extracted from one lung and expression of mRNA for catalase (CAT), manganese superoxide dismutase (MnSOD), copper-zinc superoxide dismutase (CuZnSOD), glutathione peroxidase (GPX), and metallothionein (MT) was determined by Northern blots and dot blots. The anatomical distribution of expression of these genes was determined by in situ hybridization studies on sections of the contralateral lung. We found that expression of both MnSOD and MT was significantly increased (to levels 70 to 400% greater than in controls) at days 1 and 2 and returned to control levels by day 7. GPX expression was slightly but significantly increased at days 7 and 14 in smoke-exposed animals. CuZnSOD and CAT expression did not change from control levels. In control lungs, MnSOD was expressed in all cell types, with the highest expression seen in bronchial epithelial cells; a notable finding was a mosaic pattern of expression in the bronchial epithelium, with contiguous areas of bronchial epithelium composed of cells expressing MnSOD at high levels (hot spots), compared with the adjacent epithelium. In smoke-exposed lungs, the hot spots became less prominent after 1 and 2 days of exposure to smoke, but after 7 and 14 days the distribution of MnSOD expression was similar in control and smoke-exposed animals. CAT, CuZnSOD, GPX, and MT also showed widespread expression in the lung by in situ hybridization; GPX, CuZnSOD and MT were all most highly expressed in bronchial epithelium, whereas CAT expression levels were similar in all cell types. In contrast to MnSOD, expression of CAT, CuZnSOD, GPX, and MT was uniform within the bronchial epithelium, and the distribution of expression was the same in control and smoke-exposed animals at all time points. We conclude that most of these antioxidant enzymes and scavengers show prominent bronchial expression but that MnSOD shows a unique pattern, with intense hot spots in the epithelium of the small airways. This pattern is similar to the phenomenon of clonal heterogeneity described in other tissues but not previously reported in the lung. We conclude that cigarette smoke, like other forms of oxidant attack, transiently increases expression of MnSOD, and up-regulation of MnSOD expression appears to occur particularly in bronchial epithelial cells, which normally express MnSOD at relatively low levels. MT expression is also transiently increased by smoke whereas GPX expression increases after prolonged (7 to 14 days) exposure to cigarette smoke.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Birren B. W., Ganz T., Piletz J. E., Herschman H. R. Molecular cloning of the rat metallothionein 1 (MT-1) mRNA sequence. DNA. 1983;2(1):15–22. doi: 10.1089/dna.1.1983.2.15. [DOI] [PubMed] [Google Scholar]
- Bradl M., Larue L., Mintz B. Clonal coat color variation due to a transforming gene expressed in melanocytes of transgenic mice. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6447–6451. doi: 10.1073/pnas.88.15.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chubatsu L. S., Meneghini R. Metallothionein protects DNA from oxidative damage. Biochem J. 1993 Apr 1;291(Pt 1):193–198. doi: 10.1042/bj2910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churg A., Keeling B., Gilks B., Porter S., Olive P. Rat mesothelial and tracheal epithelial cells show equal DNA sensitivity to hydrogen peroxide-induced oxidant injury. Am J Physiol. 1995 May;268(5 Pt 1):L832–L838. doi: 10.1152/ajplung.1995.268.5.L832. [DOI] [PubMed] [Google Scholar]
- Clerch L. B., Massaro D. Tolerance of rats to hyperoxia. Lung antioxidant enzyme gene expression. J Clin Invest. 1993 Feb;91(2):499–508. doi: 10.1172/JCI116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde B. L., Chang L. Y., Auten R. L., Ho Y. S., Crapo J. D. Distribution of manganese superoxide dismutase mRNA in normal and hyperoxic rat lung. Am J Respir Cell Mol Biol. 1993 May;8(5):530–537. doi: 10.1165/ajrcmb/8.5.530. [DOI] [PubMed] [Google Scholar]
- Cross C. E., van der Vliet A., O'Neill C. A., Eiserich J. P. Reactive oxygen species and the lung. Lancet. 1994 Oct 1;344(8927):930–933. doi: 10.1016/s0140-6736(94)92275-6. [DOI] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L., Huber G. L. The effects of experimental exposure to tobacco smoke on the oxidative metabolism of alveolar macrophages. J Reticuloendothel Soc. 1979 Jun;25(6):597–604. [PubMed] [Google Scholar]
- Furuta S., Hayashi H., Hijikata M., Miyazawa S., Osumi T., Hashimoto T. Complete nucleotide sequence of cDNA and deduced amino acid sequence of rat liver catalase. Proc Natl Acad Sci U S A. 1986 Jan;83(2):313–317. doi: 10.1073/pnas.83.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M. P., Khanduja K. L., Sharma R. R. Effect of cigarette smoke inhalation on antioxidant enzymes and lipid peroxidation in the rat. Toxicol Lett. 1988 May;41(2):107–114. doi: 10.1016/0378-4274(88)90084-7. [DOI] [PubMed] [Google Scholar]
- Hilbert J., Mohsenin V. Adaptation of lung antioxidants to cigarette smoking in humans. Chest. 1996 Oct;110(4):916–920. doi: 10.1378/chest.110.4.916. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Crapo J. D. Nucleotide sequences of cDNAs coding for rat manganese-containing superoxide dismutase. Nucleic Acids Res. 1987 Dec 10;15(23):10070–10070. doi: 10.1093/nar/15.23.10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. S., Crapo J. D. cDNA and deduced amino acid sequence of rat copper-zinc-containing superoxide dismutase. Nucleic Acids Res. 1987 Aug 25;15(16):6746–6746. doi: 10.1093/nar/15.16.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen Y. M., Marsh J. P., Absher M. P., Hemenway D., Vacek P. M., Leslie K. O., Borm P. J., Mossman B. T. Expression of antioxidant enzymes in rat lungs after inhalation of asbestos or silica. J Biol Chem. 1992 May 25;267(15):10625–10630. [PubMed] [Google Scholar]
- Janssen Y. M., Van Houten B., Borm P. J., Mossman B. T. Cell and tissue responses to oxidative damage. Lab Invest. 1993 Sep;69(3):261–274. [PubMed] [Google Scholar]
- Kilburn K. H., McKenzie W. Leukocyte recruitment to airways by cigarette smoke and particle phase in contrast to cytotoxicity of vapor. Science. 1975 Aug 22;189(4203):634–637. doi: 10.1126/science.1162344. [DOI] [PubMed] [Google Scholar]
- Kinnula V. L., Crapo J. D., Raivio K. O. Generation and disposal of reactive oxygen metabolites in the lung. Lab Invest. 1995 Jul;73(1):3–19. [PubMed] [Google Scholar]
- Lee C. Y., Pastore J. N., Tang G., Tsan M. F. Cellular distribution of pulmonary Mn and CuZn superoxide dismutase: effect of hyperoxia and interleukin-1. J Histochem Cytochem. 1994 Sep;42(9):1201–1205. doi: 10.1177/42.9.8064127. [DOI] [PubMed] [Google Scholar]
- Lewis-Molock Y., Suzuki K., Taniguchi N., Nguyen D. H., Mason R. J., White C. W. Lung manganese superoxide dismutase increases during cytokine-mediated protection against pulmonary oxygen toxicity in rats. Am J Respir Cell Mol Biol. 1994 Feb;10(2):133–141. doi: 10.1165/ajrcmb.10.2.8110468. [DOI] [PubMed] [Google Scholar]
- McCusker K., Hoidal J. Selective increase of antioxidant enzyme activity in the alveolar macrophages from cigarette smokers and smoke-exposed hamsters. Am Rev Respir Dis. 1990 Mar;141(3):678–682. doi: 10.1164/ajrccm/141.3.678. [DOI] [PubMed] [Google Scholar]
- Michaelson J. Cellular selection in the genesis of multicellular organization. Lab Invest. 1993 Aug;69(2):136–151. [PubMed] [Google Scholar]
- Michiels C., Raes M., Toussaint O., Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med. 1994 Sep;17(3):235–248. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Mintz B. Clonal basis of mammalian differentiation. Symp Soc Exp Biol. 1971;25:345–370. [PubMed] [Google Scholar]
- Mintz B. Gene control of mammalian pigmentary differentiation. I. Clonal origin of melanocytes. Proc Natl Acad Sci U S A. 1967 Jul;58(1):344–351. doi: 10.1073/pnas.58.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedboeuf B., Johnston C. J., Watkins R. H., Hudak B. B., Lazo J. S., Cherian M. G., Horowitz S. Increased expression of tissue inhibitor of metalloproteinases (TIMP-I) and metallothionein in murine lungs after hyperoxic exposure. Am J Respir Cell Mol Biol. 1994 Feb;10(2):123–132. doi: 10.1165/ajrcmb.10.2.8110467. [DOI] [PubMed] [Google Scholar]
- Quinlan T., Spivack S., Mossman B. T. Regulation of antioxidant enzymes in lung after oxidant injury. Environ Health Perspect. 1994 Jun;102 (Suppl 2):79–87. doi: 10.1289/ehp.9410279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I., MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med. 1996;21(5):669–681. doi: 10.1016/0891-5849(96)00155-4. [DOI] [PubMed] [Google Scholar]
- Sato M., Sasaki M., Hojo H. Antioxidative roles of metallothionein and manganese superoxide dismutase induced by tumor necrosis factor-alpha and interleukin-6. Arch Biochem Biophys. 1995 Feb 1;316(2):738–744. doi: 10.1006/abbi.1995.1098. [DOI] [PubMed] [Google Scholar]
- Sekhon H. S., Wright J. L., Churg A. Cigarette smoke causes rapid cell proliferation in small airways and associated pulmonary arteries. Am J Physiol. 1994 Nov;267(5 Pt 1):L557–L563. doi: 10.1152/ajplung.1994.267.5.L557. [DOI] [PubMed] [Google Scholar]
- Sen C. K., Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996 May;10(7):709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- White C. W., Avraham K. B., Shanley P. F., Groner Y. Transgenic mice with expression of elevated levels of copper-zinc superoxide dismutase in the lungs are resistant to pulmonary oxygen toxicity. J Clin Invest. 1991 Jun;87(6):2162–2168. doi: 10.1172/JCI115249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wispé J. R., Warner B. B., Clark J. C., Dey C. R., Neuman J., Glasser S. W., Crapo J. D., Chang L. Y., Whitsett J. A. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992 Nov 25;267(33):23937–23941. [PubMed] [Google Scholar]
- Wright J. L., Churg A. Cigarette smoke causes physiologic and morphologic changes of emphysema in the guinea pig. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1422–1428. doi: 10.1164/ajrccm/142.6_Pt_1.1422. [DOI] [PubMed] [Google Scholar]
- York G. K., Peirce T. H., Schwartz L. W., Cross C. E. Stimulation by cigarette smoke of glutathione peroxidase system enzyme activities in rat lung. Arch Environ Health. 1976 Nov-Dec;31(6):286–290. doi: 10.1080/00039896.1976.10667237. [DOI] [PubMed] [Google Scholar]