Abstract
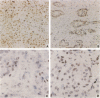
PLK (polo-like kinase) belongs to a family of serine/threonine kinases and represents the human counterpart of polo in Drosophila melanogaster and of CDC5 in Saccharomyces cerevisiae. It is strongly involved in spindle formation and chromosome segregation during mitosis. We have shown previously that PLK mRNA expression correlates with the mitotic activity of cells and the prognosis of lung cancer patients. In this report, the level of PLK protein was analyzed using immunohistochemical techniques. PLK protein was found expressed in the nuclei of tumor cells from lung and breast cancer as well as in several tumor cell lines. Furthermore, in peripheral lymphocytes treated with phytohemagglutinin, elevated proliferative activity of the cells correlated with the up-regulation of PLK protein expression. In contrast, in U937 and HL-60 cells after induction of differentiation with phorbol ester, PLK immunostaining disappeared under conditions of terminal differentiation. Most of the PLK protein was found in the nucleus of proliferating cells with diffuse but distinct staining also in the cytoplasm. Taken together, high levels of PLK protein are associated with cellular proliferation. Combined with other proliferative and oncogene markers, PLK may be useful for improved prediction of the clinical prognosis of cancer patients and for early cancer diagnosis. Due to its activity late in the cell cycle, it may be a target for cancer chemotherapy.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho R., Haapasalo H., Alanen K., Haltia M., Paetau A., Kalimo H. Proliferative activity and DNA index do not significantly predict survival in primary central nervous system lymphoma. J Neuropathol Exp Neurol. 1995 Nov;54(6):826–832. doi: 10.1097/00005072-199511000-00009. [DOI] [PubMed] [Google Scholar]
- Bräuninger A., Strebhardt K., Rübsamen-Waigmann H. Identification and functional characterization of the human and murine polo-like kinase (Plk) promoter. Oncogene. 1995 Nov 2;11(9):1793–1800. [PubMed] [Google Scholar]
- FLIEDNER T., KESSE M., CRONKITE E. P., ROBERTSON J. S. CELL PROLIFERATION IN GERMINAL CENTERS OF THE RAT SPLEEN. Ann N Y Acad Sci. 1964 Feb 28;113:578–594. doi: 10.1111/j.1749-6632.1964.tb40692.x. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Golsteyn R. M., Mundt K. E., Fry A. M., Nigg E. A. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol. 1995 Jun;129(6):1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golsteyn R. M., Schultz S. J., Bartek J., Ziemiecki A., Ried T., Nigg E. A. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci. 1994 Jun;107(Pt 6):1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- Hamanaka R., Maloid S., Smith M. R., O'Connell C. D., Longo D. L., Ferris D. K. Cloning and characterization of human and murine homologues of the Drosophila polo serine-threonine kinase. Cell Growth Differ. 1994 Mar;5(3):249–257. [PubMed] [Google Scholar]
- Holtrich U., Wolf G., Bräuninger A., Karn T., Böhme B., Rübsamen-Waigmann H., Strebhardt K. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1736–1740. doi: 10.1073/pnas.91.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshgegian A. A., Cnaan A. Proliferation markers in breast carcinoma. Mitotic figure count, S-phase fraction, proliferating cell nuclear antigen, Ki-67 and MIB-1. Am J Clin Pathol. 1995 Jul;104(1):42–49. doi: 10.1093/ajcp/104.1.42. [DOI] [PubMed] [Google Scholar]
- Kitada K., Johnson A. L., Johnston L. H., Sugino A. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol Cell Biol. 1993 Jul;13(7):4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Yuan Y. L., Kuriyama R., Erikson R. L. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol Cell Biol. 1995 Dec;15(12):7143–7151. doi: 10.1128/mcb.15.12.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamazares S., Moreira A., Tavares A., Girdham C., Spruce B. A., Gonzalez C., Karess R. E., Glover D. M., Sunkel C. E. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991 Dec;5(12A):2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- Mitrou P. S., Queisser W., Lennert K., Sandritter W. Kombinierte autoradiographisch-cytophotometrische Untersuchungen von Keimzentrumszellen der menschlichen Tonsille. Virchows Arch B Cell Pathol. 1969;3(2):156–170. [PubMed] [Google Scholar]
- Ng I. O., Na J., Lai E. C., Fan S. T., Ng M. Ki-67 antigen expression in hepatocellular carcinoma using monoclonal antibody MIB1. A comparison with proliferating cell nuclear antigen. Am J Clin Pathol. 1995 Sep;104(3):313–318. doi: 10.1093/ajcp/104.3.313. [DOI] [PubMed] [Google Scholar]
- PILGRIM C., ERB W., MAURER W. DIURNAL FLUCTUATIONS IN THE NUMBERS OF DNA SYNTHESIZING NUCLEI IN VARIOUS MOUSE TISSUES. Nature. 1963 Aug 31;199:863–863. doi: 10.1038/199863a0. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Gattuso P., Aranha G., Carson H. J. Cell proliferation markers in predicting metastases in malignant melanoma. J Cutan Pathol. 1995 Jun;22(3):248–251. doi: 10.1111/j.1600-0560.1995.tb00746.x. [DOI] [PubMed] [Google Scholar]
- Rübsamen-Waigmann H., Becker W. B., Helm E. B., Brodt R., Fischer H., Henco K., Brede H. D. Isolation of variants of lymphocytopathic retroviruses from the peripheral blood and cerebrospinal fluid of patients with ARC or AIDS. J Med Virol. 1986 Aug;19(4):335–344. doi: 10.1002/jmv.1890190406. [DOI] [PubMed] [Google Scholar]
- Sun X. F., Carstensen J. M., Stål O., Zhang H., Nordenskjöld B. Proliferating cell nuclear antigen (PCNA) in relation to ras, c-erbB-2,p53, clinico-pathological variables and prognosis in colorectal adenocarcinoma. Int J Cancer. 1996 Feb 20;69(1):5–8. doi: 10.1002/(SICI)1097-0215(19960220)69:1<5::AID-IJC2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Weikel W., Brumm C., Wilkens C., Beck T., Knapstein P. G. Growth fractions (Ki-67) in primary breast cancers, with particular reference to node-negative tumors. Cancer Detect Prev. 1995;19(5):446–450. [PubMed] [Google Scholar]
- Wolf G., Elez R., Doermer A., Holtrich U., Ackermann H., Stutte H. J., Altmannsberger H. M., Rübsamen-Waigmann H., Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997 Feb 6;14(5):543–549. doi: 10.1038/sj.onc.1200862. [DOI] [PubMed] [Google Scholar]
- Yu C. C., Dublin E. A., Camplejohn R. S., Levison D. A. Optimization of immunohistochemical staining of proliferating cells in paraffin sections of breast carcinoma using antibodies to proliferating cell nuclear antigen and the Ki-67 antigen. Anal Cell Pathol. 1995 Jul;9(1):45–52. [PubMed] [Google Scholar]