Abstract
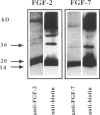
Fibroblast growth factors (FGFs) play multiple roles during development and in adult tissues as paracrine regulators of growth and differentiation. FGFs signal through transmembrane receptor tyrosine kinases, but heparan sulfate is also required for signaling by members of the FGF family. In addition, heparan sulfate may be involved in determining tissue distribution of FGFs. Using biotinylated FGF-2 and FGF-7 (KGF) as probes, we have identified specific interactions between FGFs and heparan sulfates in human tissues. Both FGF species bind to tissue mast cells and to epithelial cell membranes. Binding to basement membrane heparan sulfate is tissue source dependent and specific. Although FGF-2 strongly binds to basement membrane heparan sulfate in skin and most other tissue sites examined, FGF-7 fails to bind to basement membrane heparan sulfate in most locations. However, in subendothelial matrix in blood vessels and in the basement membrane of a papillary renal cell carcinoma, strong FGF-7 binding is seen. In summary, distinct and specific affinities of heparan sulfates for different FGFs were identified that may affect growth factor activation and local distribution. Heparan sulfate may have a gatekeeper function to either restrict or permit diffusion of heparin-binding growth factors across the basement membrane.
Full text
PDF




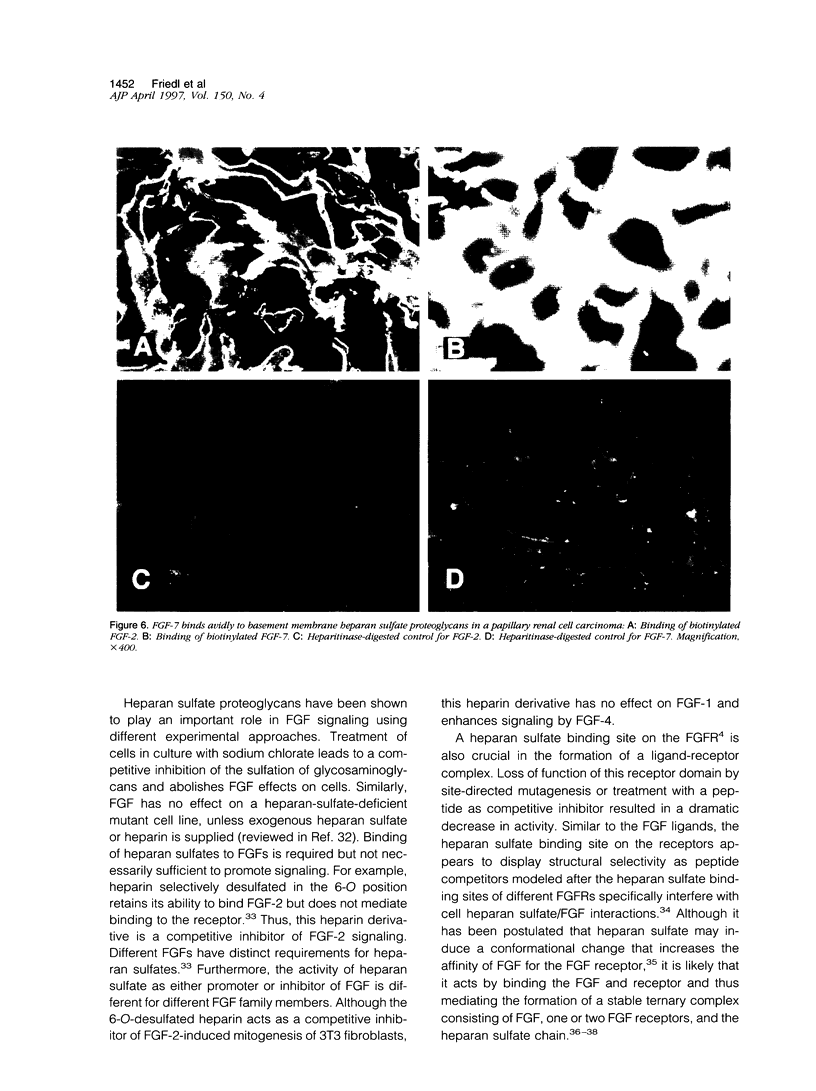



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Bottaro D. P., Miki T., Ron D., Finch P. W., Fleming T. P., Ahn J., Taylor W. G., Rubin J. S. Keratinocyte growth factor. A fibroblast growth factor family member with unusual target cell specificity. Ann N Y Acad Sci. 1991;638:62–77. doi: 10.1111/j.1749-6632.1991.tb49018.x. [DOI] [PubMed] [Google Scholar]
- Alarid E. T., Rubin J. S., Young P., Chedid M., Ron D., Aaronson S. A., Cunha G. R. Keratinocyte growth factor functions in epithelial induction during seminal vesicle development. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1074–1078. doi: 10.1073/pnas.91.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviezer D., Hecht D., Safran M., Eisinger M., David G., Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994 Dec 16;79(6):1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Bennett K. L., Jackson D. G., Simon J. C., Tanczos E., Peach R., Modrell B., Stamenkovic I., Plowman G., Aruffo A. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol. 1995 Feb;128(4):687–698. doi: 10.1083/jcb.128.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan M., Mohsen M., Levi E., Wygoda M. R., Miao H. Q., Lider O., Svahn C. M., Ekre H. P., Ishai-Michaeli R., Bar-Shavit R. Structural requirements for inhibition of melanoma lung colonization by heparanase inhibiting species of heparin. Isr J Med Sci. 1995 Feb-Mar;31(2-3):106–118. [PubMed] [Google Scholar]
- Brickman Y. G., Ford M. D., Small D. H., Bartlett P. F., Nurcombe V. Heparan sulfates mediate the binding of basic fibroblast growth factor to a specific receptor on neural precursor cells. J Biol Chem. 1995 Oct 20;270(42):24941–24948. doi: 10.1074/jbc.270.42.24941. [DOI] [PubMed] [Google Scholar]
- Chernousov M. A., Carey D. J. N-syndecan (syndecan 3) from neonatal rat brain binds basic fibroblast growth factor. J Biol Chem. 1993 Aug 5;268(22):16810–16814. [PubMed] [Google Scholar]
- Cordon-Cardo C., Vlodavsky I., Haimovitz-Friedman A., Hicklin D., Fuks Z. Expression of basic fibroblast growth factor in normal human tissues. Lab Invest. 1990 Dec;63(6):832–840. [PubMed] [Google Scholar]
- Danilenko D. M., Ring B. D., Yanagihara D., Benson W., Wiemann B., Starnes C. O., Pierce G. F. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 1995 Jul;147(1):145–154. [PMC free article] [PubMed] [Google Scholar]
- David G., Bai X. M., Van der Schueren B., Cassiman J. J., Van den Berghe H. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J Cell Biol. 1992 Nov;119(4):961–975. doi: 10.1083/jcb.119.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. J., McCaffrey T. A., Haimovitz-Friedman A., Vergilio J. A., Nicholson A. C. Macrophage and foam cell release of matrix-bound growth factors. Role of plasminogen activation. J Biol Chem. 1993 Jun 5;268(16):11951–11958. [PubMed] [Google Scholar]
- Ford M. D., Bartlett P. F., Nurcombe V. Co-localization of FGF-2 and a novel heparan sulphate proteoglycan in embryonic mouse brain. Neuroreport. 1994 Jan 31;5(5):565–568. doi: 10.1097/00001756-199401000-00008. [DOI] [PubMed] [Google Scholar]
- Guimond S., Maccarana M., Olwin B. B., Lindahl U., Rapraeger A. C. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J Biol Chem. 1993 Nov 15;268(32):23906–23914. [PubMed] [Google Scholar]
- Guo L., Degenstein L., Fuchs E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996 Jan 15;10(2):165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- Guo L., Yu Q. C., Fuchs E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 1993 Mar;12(3):973–986. doi: 10.1002/j.1460-2075.1993.tb05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. D., Allen-Hoffmann B. L. Keratinocyte growth factor inhibits cross-linked envelope formation and nucleosomal fragmentation in cultured human keratinocytes. J Biol Chem. 1996 Mar 15;271(11):6245–6251. doi: 10.1074/jbc.271.11.6245. [DOI] [PubMed] [Google Scholar]
- Hughes S. E., Hall P. A. Immunolocalization of fibroblast growth factor receptor 1 and its ligands in human tissues. Lab Invest. 1993 Aug;69(2):173–182. [PubMed] [Google Scholar]
- Jaye M., Schlessinger J., Dionne C. A. Fibroblast growth factor receptor tyrosine kinases: molecular analysis and signal transduction. Biochim Biophys Acta. 1992 Jun 10;1135(2):185–199. doi: 10.1016/0167-4889(92)90136-y. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Williams L. T. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Kan M., Wang F., Xu J., Crabb J. W., Hou J., McKeehan W. L. An essential heparin-binding domain in the fibroblast growth factor receptor kinase. Science. 1993 Mar 26;259(5103):1918–1921. doi: 10.1126/science.8456318. [DOI] [PubMed] [Google Scholar]
- Kessler D. A., Langer R. S., Pless N. A., Folkman J. Mast cells and tumor angiogenesis. Int J Cancer. 1976 Nov 15;18(5):703–709. doi: 10.1002/ijc.2910180520. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1989;1(4):207–235. doi: 10.1016/0955-2235(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Koji T., Chedid M., Rubin J. S., Slayden O. D., Csaky K. G., Aaronson S. A., Brenner R. M. Progesterone-dependent expression of keratinocyte growth factor mRNA in stromal cells of the primate endometrium: keratinocyte growth factor as a progestomedin. J Cell Biol. 1994 Apr;125(2):393–401. doi: 10.1083/jcb.125.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRochelle W. J., Dirsch O. R., Finch P. W., Cheon H. G., May M., Marchese C., Pierce J. H., Aaronson S. A. Specific receptor detection by a functional keratinocyte growth factor-immunoglobulin chimera. J Cell Biol. 1995 Apr;129(2):357–366. doi: 10.1083/jcb.129.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I. J. The ins and outs of fibroblast growth factors. Cell. 1994 Aug 26;78(4):547–552. doi: 10.1016/0092-8674(94)90520-7. [DOI] [PubMed] [Google Scholar]
- Mathieu M., Chatelain E., Ornitz D., Bresnick J., Mason I., Kiefer P., Dickson C. Receptor binding and mitogenic properties of mouse fibroblast growth factor 3. Modulation of response by heparin. J Biol Chem. 1995 Oct 13;270(41):24197–24203. doi: 10.1074/jbc.270.41.24197. [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Dikic I., Sorokin A., Burgess W. H., Jaye M., Schlessinger J. Identification of six novel autophosphorylation sites on fibroblast growth factor receptor 1 and elucidation of their importance in receptor activation and signal transduction. Mol Cell Biol. 1996 Mar;16(3):977–989. doi: 10.1128/mcb.16.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D. High and low affinity binding sites for basic fibroblast growth factor on cultured cells: absence of a role for low affinity binding in the stimulation of plasminogen activator production by bovine capillary endothelial cells. J Cell Physiol. 1987 Apr;131(1):123–130. doi: 10.1002/jcp.1041310118. [DOI] [PubMed] [Google Scholar]
- Nurcombe V., Ford M. D., Wildschut J. A., Bartlett P. F. Developmental regulation of neural response to FGF-1 and FGF-2 by heparan sulfate proteoglycan. Science. 1993 Apr 2;260(5104):103–106. doi: 10.1126/science.7682010. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Yayon A., Flanagan J. G., Svahn C. M., Levi E., Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992 Jan;12(1):240–247. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoliano M. W., Horlick R. A., Springer B. A., Van Dyk D. E., Tobery T., Wetmore D. R., Lear J. D., Nahapetian A. T., Bradley J. D., Sisk W. P. Multivalent ligand-receptor binding interactions in the fibroblast growth factor system produce a cooperative growth factor and heparin mechanism for receptor dimerization. Biochemistry. 1994 Aug 30;33(34):10229–10248. doi: 10.1021/bi00200a003. [DOI] [PubMed] [Google Scholar]
- Prestrelski S. J., Fox G. M., Arakawa T. Binding of heparin to basic fibroblast growth factor induces a conformational change. Arch Biochem Biophys. 1992 Mar;293(2):314–319. doi: 10.1016/0003-9861(92)90401-h. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Guimond S., Krufka A., Olwin B. B. Regulation by heparan sulfate in fibroblast growth factor signaling. Methods Enzymol. 1994;245:219–240. doi: 10.1016/0076-6879(94)45013-7. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Reed J. A., Albino A. P., McNutt N. S. Human cutaneous mast cells express basic fibroblast growth factor. Lab Invest. 1995 Feb;72(2):215–222. [PubMed] [Google Scholar]
- Reich-Slotky R., Bonneh-Barkay D., Shaoul E., Bluma B., Svahn C. M., Ron D. Differential effect of cell-associated heparan sulfates on the binding of keratinocyte growth factor (KGF) and acidic fibroblast growth factor to the KGF receptor. J Biol Chem. 1994 Dec 23;269(51):32279–32285. [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Salmivirta M., Heino J., Jalkanen M. Basic fibroblast growth factor-syndecan complex at cell surface or immobilized to matrix promotes cell growth. J Biol Chem. 1992 Sep 5;267(25):17606–17610. [PubMed] [Google Scholar]
- Schulze-Osthoff K., Risau W., Vollmer E., Sorg C. In situ detection of basic fibroblast growth factor by highly specific antibodies. Am J Pathol. 1990 Jul;137(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- Spivak-Kroizman T., Lemmon M. A., Dikic I., Ladbury J. E., Pinchasi D., Huang J., Jaye M., Crumley G., Schlessinger J., Lax I. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell. 1994 Dec 16;79(6):1015–1024. doi: 10.1016/0092-8674(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Springer B. A., Pantoliano M. W., Barbera F. A., Gunyuzlu P. L., Thompson L. D., Herblin W. F., Rosenfeld S. A., Book G. W. Identification and concerted function of two receptor binding surfaces on basic fibroblast growth factor required for mitogenesis. J Biol Chem. 1994 Oct 28;269(43):26879–26884. [PubMed] [Google Scholar]
- Steinfeld R., Van Den Berghe H., David G. Stimulation of fibroblast growth factor receptor-1 occupancy and signaling by cell surface-associated syndecans and glypican. J Cell Biol. 1996 Apr;133(2):405–416. doi: 10.1083/jcb.133.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. D., Pantoliano M. W., Springer B. A. Energetic characterization of the basic fibroblast growth factor-heparin interaction: identification of the heparin binding domain. Biochemistry. 1994 Apr 5;33(13):3831–3840. doi: 10.1021/bi00179a006. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Yi E. S., Cardiff R., Yin S., Bikhazi N., Biltz R., Morris C. F., Pierce G. F. Keratinocyte growth factor is a growth factor for mammary epithelium in vivo. The mammary epithelium of lactating rats is resistant to the proliferative action of keratinocyte growth factor. Am J Pathol. 1994 May;144(5):862–868. [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Eldor A., Haimovitz-Friedman A., Matzner Y., Ishai-Michaeli R., Lider O., Naparstek Y., Cohen I. R., Fuks Z. Expression of heparanase by platelets and circulating cells of the immune system: possible involvement in diapedesis and extravasation. Invasion Metastasis. 1992;12(2):112–127. [PubMed] [Google Scholar]
- Vlodavsky I., Mohsen M., Lider O., Svahn C. M., Ekre H. P., Vigoda M., Ishai-Michaeli R., Peretz T. Inhibition of tumor metastasis by heparanase inhibiting species of heparin. Invasion Metastasis. 1994;14(1-6):290–302. [PubMed] [Google Scholar]
- Weidner N., Semple J. P., Welch W. R., Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991 Jan 3;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Werner S., Duan D. S., de Vries C., Peters K. G., Johnson D. E., Williams L. T. Differential splicing in the extracellular region of fibroblast growth factor receptor 1 generates receptor variants with different ligand-binding specificities. Mol Cell Biol. 1992 Jan;12(1):82–88. doi: 10.1128/mcb.12.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Peters K. G., Longaker M. T., Fuller-Pace F., Banda M. J., Williams L. T. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Smola H., Liao X., Longaker M. T., Krieg T., Hofschneider P. H., Williams L. T. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994 Nov 4;266(5186):819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- Whitelock J. M., Murdoch A. D., Iozzo R. V., Underwood P. A. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996 Apr 26;271(17):10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]