Abstract
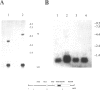
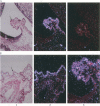
The His-1 gene is expressed as a 3-kb spliced and polyadenylated RNA that is believed to function in the absence of an encoded protein. The precise function of the His-1 gene is unknown, but its transcriptional activation in a series of mouse leukemias has implicated the His-1 RNA in leukemogenesis when it is abnormally expressed. To study the oncogenic potential of this gene in more detail, we have examined the normal tissue distribution of His-1 RNA during mouse embryogenesis and in various adult tissues. His-1 expression was detected at low levels in the epithelia of the adult mouse stomach, prostate, seminal vesicle, and the developing choroid plexus by in situ hybridization. All other tissues examined lacked detectable levels of hybridizing RNA, suggesting that normal His-1 gene expression is highly restricted to these epithelial sites. These transcripts were not detectable by Northern blot analysis of normal tissues but were readily identified in five mouse leukemias and in five carcinomas of the choroid plexus. These data indicate that the His-1 gene expression is highly restricted and suggest that inappropriate activation of this gene may contribute to carcinogenesis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Askew D. S., Bartholomew C., Buchberg A. M., Valentine M. B., Jenkins N. A., Copeland N. G., Ihle J. N. His-1 and His-2: identification and chromosomal mapping of two commonly rearranged sites of viral integration in a myeloid leukemia. Oncogene. 1991 Nov;6(11):2041–2047. [PubMed] [Google Scholar]
- Askew D. S., Bartholomew C., Ihle J. N. Insertional mutagenesis and the transformation of hematopoietic stem cells. Hematol Pathol. 1993;7(1):1–22. [PubMed] [Google Scholar]
- Askew D. S., Li J., Ihle J. N. Retroviral insertions in the murine His-1 locus activate the expression of a novel RNA that lacks an extensive open reading frame. Mol Cell Biol. 1994 Mar;14(3):1743–1751. doi: 10.1128/mcb.14.3.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azumi J. I., Sachs L. Chromosome mapping of the genes that control differentiation and malignancy in myeloid leukemic cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):253–257. doi: 10.1073/pnas.74.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badley J. E., Bishop G. A., St John T., Frelinger J. A. A simple, rapid method for the purification of poly A+ RNA. Biotechniques. 1988 Feb;6(2):114–116. [PubMed] [Google Scholar]
- Brannan C. I., Dees E. C., Ingram R. S., Tilghman S. M. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990 Jan;10(1):28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., McCabe V. M., Norris D. P., Cooper P. J., Swift S., Rastan S. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992 Oct 30;71(3):515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Hendrich B. D., Rupert J. L., Lafrenière R. G., Xing Y., Lawrence J., Willard H. F. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992 Oct 30;71(3):527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Brunkow M. E., Tilghman S. M. Ectopic expression of the H19 gene in mice causes prenatal lethality. Genes Dev. 1991 Jun;5(6):1092–1101. doi: 10.1101/gad.5.6.1092. [DOI] [PubMed] [Google Scholar]
- Hao Y., Crenshaw T., Moulton T., Newcomb E., Tycko B. Tumour-suppressor activity of H19 RNA. Nature. 1993 Oct 21;365(6448):764–767. doi: 10.1038/365764a0. [DOI] [PubMed] [Google Scholar]
- Katzav S., Martin-Zanca D., Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989 Aug;8(8):2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Declève A., Ricciardi-Castagnoli P., Boniver J., Finn O. J., Kaplan H. S. Establishment, characterization and virus expression of cell lines derived from radiation- and virus-induced lymphomas of C57BL/Ka mice. Int J Cancer. 1979 Aug;24(2):168–177. doi: 10.1002/ijc.2910240208. [DOI] [PubMed] [Google Scholar]
- Penny G. D., Kay G. F., Sheardown S. A., Rastan S., Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996 Jan 11;379(6561):131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- Pierga J. Y., Kalifa C., Terrier-Lacombe M. J., Habrand J. L., Lemerle J. Carcinoma of the choroid plexus: a pediatric experience. Med Pediatr Oncol. 1993;21(7):480–487. doi: 10.1002/mpo.2950210705. [DOI] [PubMed] [Google Scholar]
- Schwarzbaum S., Halpern R., Diamond B. The generation of macrophage-like cell lines by transfection with SV40 origin defective DNA. J Immunol. 1984 Mar;132(3):1158–1162. [PubMed] [Google Scholar]
- Symonds H., Krall L., Remington L., Saenz-Robles M., Lowe S., Jacks T., Van Dyke T. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994 Aug 26;78(4):703–711. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Trakhtenbrot L., Krauthgamer R., Resnitzky P., Haran-Ghera N. Deletion of chromosome 2 is an early event in the development of radiation-induced myeloid leukemia in SJL/J mice. Leukemia. 1988 Aug;2(8):545–550. [PubMed] [Google Scholar]
- Tsichlis P. N., Lazo P. A. Virus-host interactions and the pathogenesis of murine and human oncogenic retroviruses. Curr Top Microbiol Immunol. 1991;171:95–171. doi: 10.1007/978-3-642-76524-7_5. [DOI] [PubMed] [Google Scholar]
- Tycowski K. T., Shu M. D., Steitz J. A. A mammalian gene with introns instead of exons generating stable RNA products. Nature. 1996 Feb 1;379(6564):464–466. doi: 10.1038/379464a0. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. A., Finlay C., Miller D., Marks J., Lozano G., Levine A. J. Relationship between simian virus 40 large tumor antigen expression and tumor formation in transgenic mice. J Virol. 1987 Jun;61(6):2029–2032. doi: 10.1128/jvi.61.6.2029-2032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevrick R., Kerns J. A., Francke U. Identification of a novel paternally expressed gene in the Prader-Willi syndrome region. Hum Mol Genet. 1994 Oct;3(10):1877–1882. doi: 10.1093/hmg/3.10.1877. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., McMahon A. P. Expression pattern of the FGF-related proto-oncogene int-2 suggests multiple roles in fetal development. Development. 1989 Jan;105(1):131–136. doi: 10.1242/dev.105.1.131. [DOI] [PubMed] [Google Scholar]
- Witte D. P., Aronow B. J., Stauderman M. L., Stuart W. D., Clay M. A., Gruppo R. A., Jenkins S. H., Harmony J. A. Platelet activation releases megakaryocyte-synthesized apolipoprotein J, a highly abundant protein in atheromatous lesions. Am J Pathol. 1993 Sep;143(3):763–773. [PMC free article] [PubMed] [Google Scholar]
- van Lohuizen M., Berns A. Tumorigenesis by slow-transforming retroviruses--an update. Biochim Biophys Acta. 1990 Dec 11;1032(2-3):213–235. doi: 10.1016/0304-419x(90)90005-l. [DOI] [PubMed] [Google Scholar]