Abstract
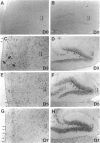
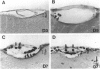
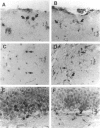
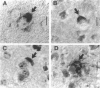
Fatal murine cerebral malaria (FMCM) is an immunopathological process. The depletion of CD4+ T cells, or the administration of antioxidants or antibodies against certain cytokines, protect the mice against cerebral complications. We previously have shown that astrocytes, microglia, and monocytes play a role in the development of FMCM, suggesting that an active immune response occurs locally within the central nervous system. We now have investigated the functional involvement of glia and monocytes in FMCM by assessing 1) the production, 2) the temporal appearance, and 3) the cellular source of cytokine mRNA and protein in the brain. Brain sections from uninfected and FMCM mice were analyzed for the presence of cytokine mRNA and protein by in situ hybridization and immunohistochemistry. Tumor necrosis factor (TNF)-alpha mRNA and protein were associated with microglia and astrocytes, monocytes, and the cerebral vascular endothelium in FMCM mice but not uninfected animals. TNF-alpha mRNA was first detected several days before the animals showed cerebral symptoms and died. Interleukin (IL)-1 beta mRNA was found in the brains of both uninfected and FMCM mice. However, IL-1 beta protein was associated only with monocytes, the meningeal vascular endothelium, and neurons in the fronto-parietal cortex in the FMCM brains. No IL-4 or IL-6 mRNAs were detected in either group. These results provide the strongest evidence to date that cytokines, in particular TNF-alpha, produced locally in the central nervous system play a role in the pathogenesis of FMCM.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Bandtlow C. E., Meyer M., Lindholm D., Spranger M., Heumann R., Thoenen H. Regional and cellular codistribution of interleukin 1 beta and nerve growth factor mRNA in the adult rat brain: possible relationship to the regulation of nerve growth factor synthesis. J Cell Biol. 1990 Oct;111(4):1701–1711. doi: 10.1083/jcb.111.4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boje K. M., Arora P. K. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992 Aug 7;587(2):250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- Butcher G. A., Garland T., Ajdukiewicz A. B., Clark I. A. Serum tumor necrosis factor associated with malaria in patients in the Solomon Islands. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):658–661. doi: 10.1016/0035-9203(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Chang-Ling T., Neill A. L., Hunt N. H. Early microvascular changes in murine cerebral malaria detected in retinal wholemounts. Am J Pathol. 1992 May;140(5):1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Chao C. C., Gekker G., Hu S., Peterson P. K. Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol. 1994 Feb 1;152(3):1246–1252. [PubMed] [Google Scholar]
- Chao C. C., Molitor T. W., Hu S. Neuroprotective role of IL-4 against activated microglia. J Immunol. 1993 Aug 1;151(3):1473–1481. [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A., Hunt N. H. Possible roles of tumor necrosis factor in the pathology of malaria. Am J Pathol. 1987 Oct;129(1):192–199. [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Ilschner S., MacMicking J. D., Cowden W. B. TNF and Plasmodium berghei ANKA-induced cerebral malaria. Immunol Lett. 1990 Aug;25(1-3):195–198. doi: 10.1016/0165-2478(90)90114-6. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Rockett K. A., Cowden W. B. Possible central role of nitric oxide in conditions clinically similar to cerebral malaria. Lancet. 1992 Oct 10;340(8824):894–896. doi: 10.1016/0140-6736(92)93295-x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Rockett K. A. The cytokine theory of human cerebral malaria. Parasitol Today. 1994 Oct;10(10):410–412. doi: 10.1016/0169-4758(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Curfs J. H., van der Meer J. W., Sauerwein R. W., Eling W. M. Low dosages of interleukin 1 protect mice against lethal cerebral malaria. J Exp Med. 1990 Nov 1;172(5):1287–1291. doi: 10.1084/jem.172.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoster E., Vanhaesebroeck B., Vandenabeele P., Grooten J., Fiers W. Generation and biological characterization of membrane-bound, uncleavable murine tumor necrosis factor. J Biol Chem. 1995 Aug 4;270(31):18473–18478. doi: 10.1074/jbc.270.31.18473. [DOI] [PubMed] [Google Scholar]
- Finley R., Weintraub J., Louis J. A., Engers H. D., Zubler R., Lambert P. H. Prevention of cerebral malaria by adoptive transfer of malaria-specific cultured T cells into mice infected with Plasmodium berghei. J Immunol. 1983 Sep;131(3):1522–1526. [PubMed] [Google Scholar]
- Ghigo D., Todde R., Ginsburg H., Costamagna C., Gautret P., Bussolino F., Ulliers D., Giribaldi G., Deharo E., Gabrielli G. Erythrocyte stages of Plasmodium falciparum exhibit a high nitric oxide synthase (NOS) activity and release an NOS-inducing soluble factor. J Exp Med. 1995 Sep 1;182(3):677–688. doi: 10.1084/jem.182.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Bieler G., Pointaire P., De Kossodo S., Tacchini-Cotier F., Vassalli P., Piguet P. F., Lambert P. H. Significance of cytokine production and adhesion molecules in malarial immunopathology. Immunol Lett. 1990 Aug;25(1-3):189–194. doi: 10.1016/0165-2478(90)90113-5. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Heremans H., Piguet P. F., Pointaire P., Lambert P. H., Billiau A., Vassalli P. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P., Lambert P. H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [PubMed] [Google Scholar]
- Grau G. E., de Kossodo S. Cerebral malaria: mediators, mechanical obstruction or more? Parasitol Today. 1994 Oct;10(10):408–409. doi: 10.1016/0169-4758(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Hu S., Sheng W. S., Peterson P. K., Chao C. C. Cytokine modulation of murine microglial cell superoxide production. Glia. 1995 Jan;13(1):45–50. doi: 10.1002/glia.440130106. [DOI] [PubMed] [Google Scholar]
- Hunt N. H., Manduci N., Thumwood C. M. Amelioration of murine cerebral malaria by dietary restriction. Parasitology. 1993 Dec;107(Pt 5):471–476. doi: 10.1017/s0031182000068049. [DOI] [PubMed] [Google Scholar]
- Janota I., Doshi B. Cerebral malaria in the United Kingdom. J Clin Pathol. 1979 Aug;32(8):769–772. doi: 10.1136/jcp.32.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunaweera N. D., Grau G. E., Gamage P., Carter R., Mendis K. N. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3200–3203. doi: 10.1073/pnas.89.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Lee J. D., Rhoades K., Economou J. S. Interleukin-4 inhibits the expression of tumour necrosis factors alpha and beta, interleukins-1 beta and -6 and interferon-gamma. Immunol Cell Biol. 1995 Feb;73(1):57–61. doi: 10.1038/icb.1995.9. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A., Olsson T., Van der Meide P. H., Holmdahl R., Klareskog L., Höjeberg B. Interferon-gamma-like immunoreactivity in certain neurons of the central and peripheral nervous system. J Neurosci Res. 1989 Nov;24(3):451–456. doi: 10.1002/jnr.490240316. [DOI] [PubMed] [Google Scholar]
- Ma N., Hunt N. H., Madigan M. C., Chan-Ling T. Correlation between enhanced vascular permeability, up-regulation of cellular adhesion molecules and monocyte adhesion to the endothelium in the retina during the development of fatal murine cerebral malaria. Am J Pathol. 1996 Nov;149(5):1745–1762. [PMC free article] [PubMed] [Google Scholar]
- Ma N., Madigan M. C., Chan-Ling T., Hunt N. H. Compromised blood-nerve barrier, astrogliosis, and myelin disruption in optic nerves during fatal murine cerebral malaria. Glia. 1997 Feb;19(2):135–151. doi: 10.1002/(sici)1098-1136(199702)19:2<135::aid-glia5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Martin P. M., O'Callaghan J. P. A direct comparison of GFAP immunocytochemistry and GFAP concentration in various regions of ethanol-fixed rat and mouse brain. J Neurosci Methods. 1995 May;58(1-2):181–192. doi: 10.1016/0165-0270(94)00175-g. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Miyagi K., Yanagisawa N., Tsukada N., Nakayama J. [Effect of intracerebral injections of tumor necrosis factor and interleukin-1]. Arerugi. 1993 Oct;42(10):1623–1627. [PubMed] [Google Scholar]
- Medana I. M., Chan-Ling T., Hunt N. H. Redistribution and degeneration of retinal astrocytes in experimental murine cerebral malaria: relationship to disruption of the blood-retinal barrier. Glia. 1996 Jan;16(1):51–64. doi: 10.1002/(SICI)1098-1136(199601)16:1<51::AID-GLIA6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Medana I. M., Hunt N. H., Chan-Ling T. Early activation of microglia in the pathogenesis of fatal murine cerebral malaria. Glia. 1997 Feb;19(2):91–103. doi: 10.1002/(sici)1098-1136(199702)19:2<91::aid-glia1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Murabe Y., Sano Y. Morphological studies on neuroglia. V. Microglial cells in the cerebral cortex of the rat, with special reference to their possible involvement in synaptic function. Cell Tissue Res. 1982;223(3):493–506. doi: 10.1007/BF00218471. [DOI] [PubMed] [Google Scholar]
- Neill A. L., Chan-Ling T., Hunt N. H. Comparisons between microvascular changes in cerebral and non-cerebral malaria in mice, using the retinal whole-mount technique. Parasitology. 1993 Dec;107(Pt 5):477–487. doi: 10.1017/s0031182000068050. [DOI] [PubMed] [Google Scholar]
- Neill A. L., Hunt N. H. Effects of endotoxin and dexamethasone on cerebral malaria in mice. Parasitology. 1995 Nov;111(Pt 4):443–454. doi: 10.1017/s003118200006594x. [DOI] [PubMed] [Google Scholar]
- Neill A. L., Hunt N. H. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology. 1992 Oct;105(Pt 2):165–175. doi: 10.1017/s0031182000074072. [DOI] [PubMed] [Google Scholar]
- Olsson T., Kristensson K., Ljungdahl A., Maehlen J., Holmdahl R., Klareskog L. Gamma-interferon-like immunoreactivity in axotomized rat motor neurons. J Neurosci. 1989 Nov;9(11):3870–3875. doi: 10.1523/JNEUROSCI.09-11-03870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuntokun B. O. Malaria and the nervous system. Afr J Med Med Sci. 1983 Sep-Dec;12(3-4):165–172. [PubMed] [Google Scholar]
- Penfold P. L., Provis J. M., Liew S. C. Human retinal microglia express phenotypic characteristics in common with dendritic antigen-presenting cells. J Neuroimmunol. 1993 Jun;45(1-2):183–191. doi: 10.1016/0165-5728(93)90179-3. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Hume D. A., Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985 Jun;15(2):313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C. R., ffrench-Mullen J. M. Interleukin-1 beta inhibits Ca2+ channel currents in hippocampal neurons through protein kinase C. Eur J Pharmacol. 1994 Jan 1;266(1):1–10. doi: 10.1016/0922-4106(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Probert L., Akassoglou K., Pasparakis M., Kontogeorgos G., Kollias G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11294–11298. doi: 10.1073/pnas.92.24.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renno T., Krakowski M., Piccirillo C., Lin J. Y., Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995 Jan 15;154(2):944–953. [PubMed] [Google Scholar]
- Rest J. R. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76(3):410–415. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Jakobsen P. H., Allen S. J., Wheeler J. G., Bennett S., Jepsen S., Greenwood B. M. Immune response to soluble exoantigens of Plasmodium falciparum may contribute to both pathogenesis and protection in clinical malaria: evidence from a longitudinal, prospective study of semi-immune African children. Eur J Immunol. 1991 Apr;21(4):1019–1025. doi: 10.1002/eji.1830210424. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Strijbos P. J. Cytokines in neurodegeneration and repair. Int J Dev Neurosci. 1995 Jun-Jul;13(3-4):179–185. doi: 10.1016/0736-5748(95)00018-c. [DOI] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Schmid A. H. Cerebral malaria. On the nature and significance of vascular changes. Eur Neurol. 1974;12(4):197–208. doi: 10.1159/000114620. [DOI] [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Taverne J., Bate C. A., Playfair J. H. Malaria exoantigens induce TNF, are toxic and are blocked by T-independent antibody. Immunol Lett. 1990 Aug;25(1-3):207–212. doi: 10.1016/0165-2478(90)90116-8. [DOI] [PubMed] [Google Scholar]
- Thumwood C. M., Hunt N. H., Clark I. A., Cowden W. B. Breakdown of the blood-brain barrier in murine cerebral malaria. Parasitology. 1988 Jun;96(Pt 3):579–589. doi: 10.1017/s0031182000080203. [DOI] [PubMed] [Google Scholar]
- Thumwood C. M., Hunt N. H., Cowden W. B., Clark I. A. Antioxidants can prevent cerebral malaria in Plasmodium berghei-infected mice. Br J Exp Pathol. 1989 Jun;70(3):293–303. [PMC free article] [PubMed] [Google Scholar]
- Zielasek J., Tausch M., Toyka K. V., Hartung H. P. Production of nitrite by neonatal rat microglial cells/brain macrophages. Cell Immunol. 1992 Apr 15;141(1):111–120. doi: 10.1016/0008-8749(92)90131-8. [DOI] [PubMed] [Google Scholar]
- de Kossodo S., Grau G. E. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J Immunol. 1993 Nov 1;151(9):4811–4820. [PubMed] [Google Scholar]