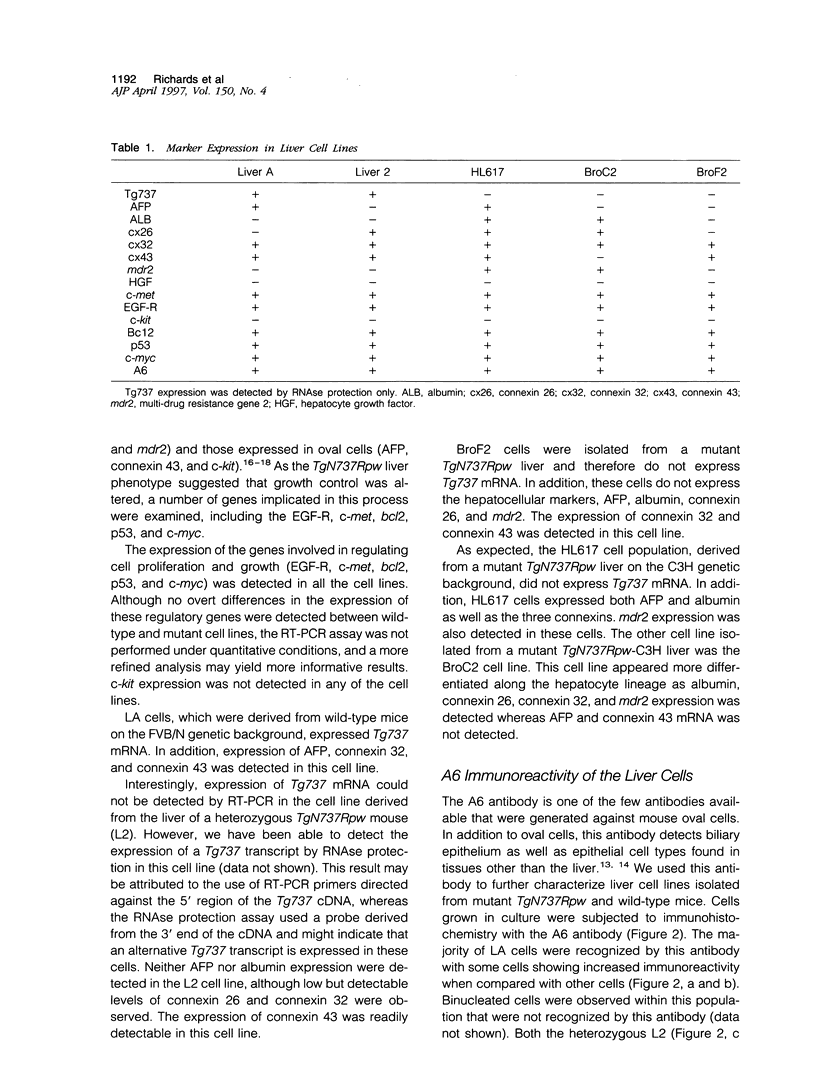
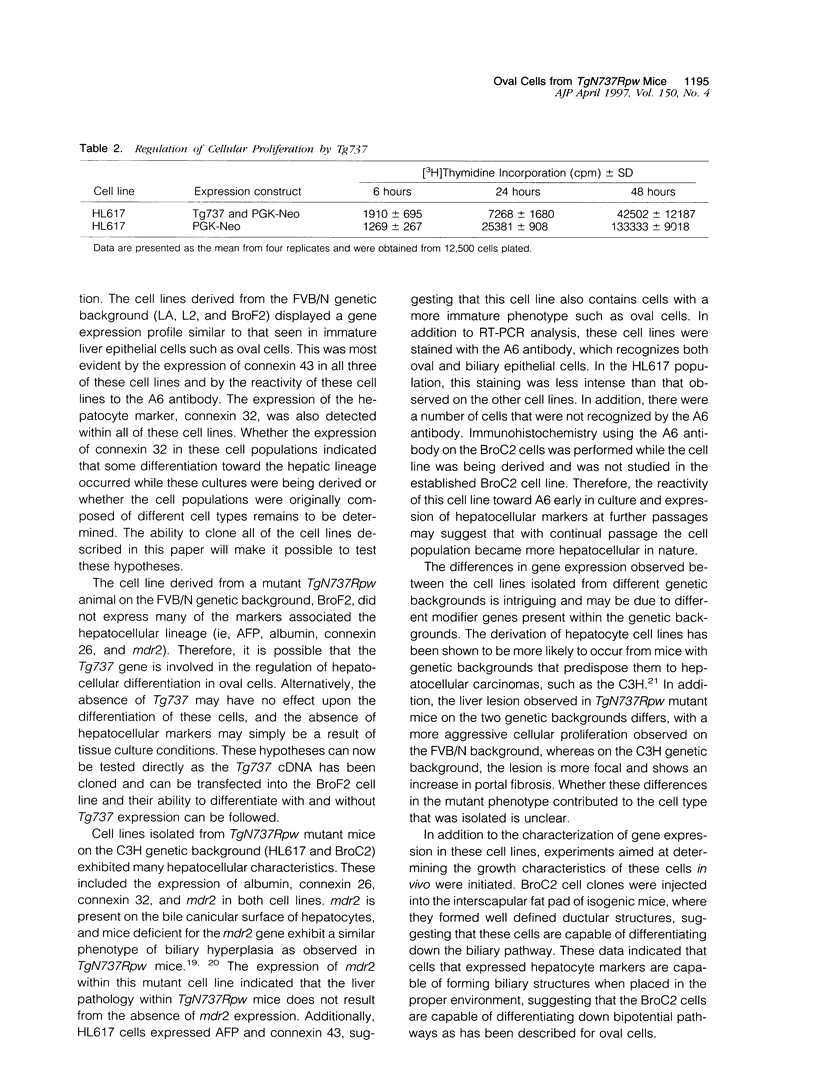
Abstract
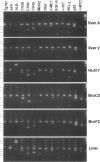
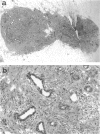
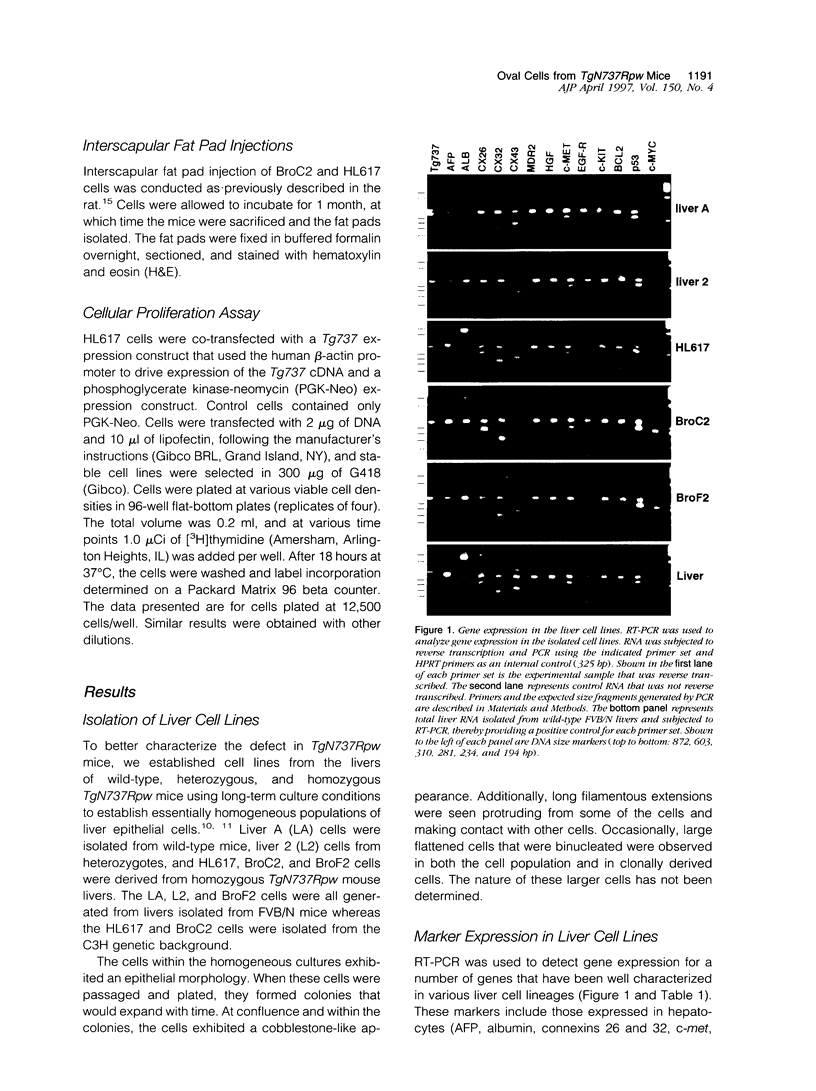
The Tg737 gene encodes a tetratricopeptide repeat containing protein that, when disrupted in TgN737Rpw mutant mice, results in pleiotropic phenotypes that include the proliferation of epithelial cells. In the kidney and liver, this causes a phenotype that resembles autosomal recessive polycystic kidney disease. In the liver, the affected epithelial cells morphologically and immunologically resemble oval cells. Here we describe the isolation, culture, and characterization of epithelial cell lines derived from the livers of wild-type, heterozygous, and homozygous TgN737Rpw mice. Essentially homogeneous cell cultures were established and the expression of liver markers was examined by reverse transcriptase polymerase chain reaction and by immunohistochemistry. All of the cell lines reacted to the A6 antibody that was raised against mouse oval cells and expressed markers seen in oval cells. Cells transplanted into the interscapular fat pads of isogenic mice formed well defined ductular structures. Furthermore, in transfection experiments, we have demonstrated the involvement of Tg737 in cellular proliferation.
Full text
PDF

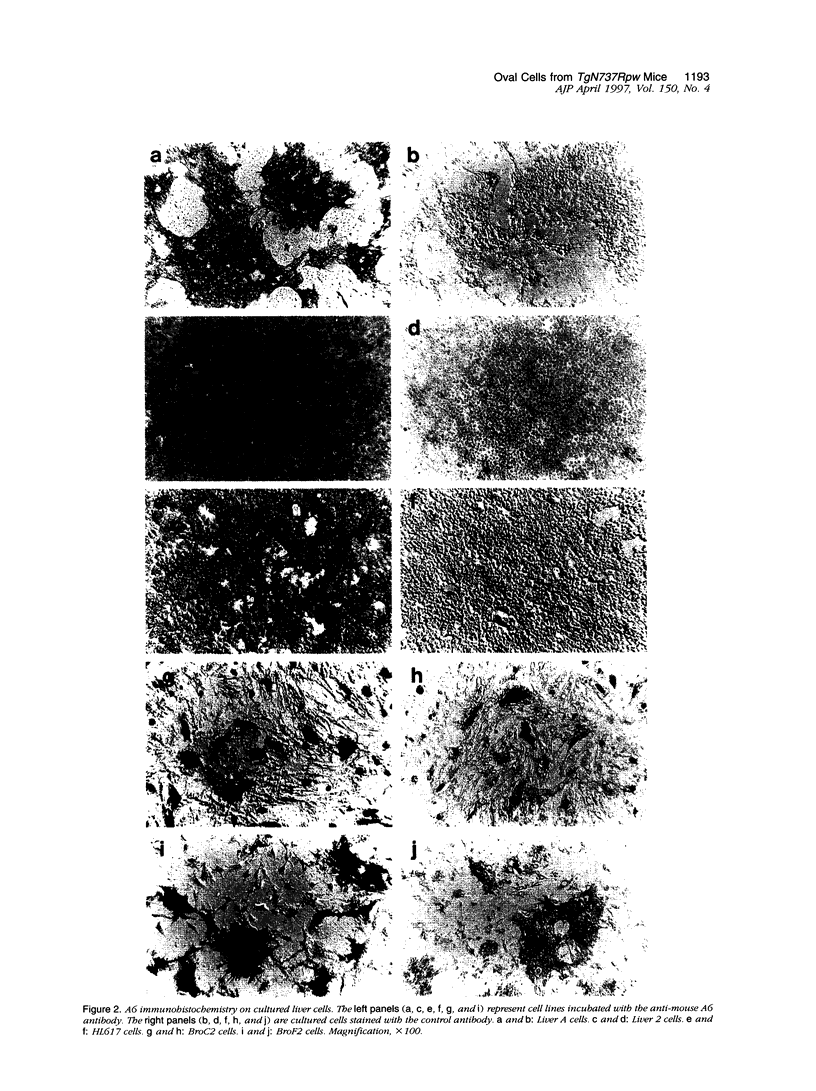



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avner E. D. Renal developmental diseases. Semin Nephrol. 1993 Sep;13(5):427–435. [PubMed] [Google Scholar]
- Avner E. D., Studnicki F. E., Young M. C., Sweeney W. E., Jr, Piesco N. P., Ellis D., Fettermann G. H. Congenital murine polycystic kidney disease. I. The ontogeny of tubular cyst formation. Pediatr Nephrol. 1987 Oct;1(4):587–596. doi: 10.1007/BF00853593. [DOI] [PubMed] [Google Scholar]
- Bernstein J. Hepatic involvement in hereditary renal syndromes. Birth Defects Orig Artic Ser. 1987;23(1):115–130. [PubMed] [Google Scholar]
- D'Agata I. D., Jonas M. M., Perez-Atayde A. R., Guay-Woodford L. M. Combined cystic disease of the liver and kidney. Semin Liver Dis. 1994 Aug;14(3):215–228. doi: 10.1055/s-2007-1007313. [DOI] [PubMed] [Google Scholar]
- Engelhardt N. V., Factor V. M., Medvinsky A. L., Baranov V. N., Lazareva M. N., Poltoranina V. S. Common antigen of oval and biliary epithelial cells (A6) is a differentiation marker of epithelial and erythroid cell lineages in early development of the mouse. Differentiation. 1993 Dec;55(1):19–26. doi: 10.1111/j.1432-0436.1993.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Engelhardt N. V., Factor V. M., Yasova A. K., Poltoranina V. S., Baranov V. N., Lasareva M. N. Common antigens of mouse oval and biliary epithelial cells. Expression on newly formed hepatocytes. Differentiation. 1990 Oct;45(1):29–37. doi: 10.1111/j.1432-0436.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Frohman M. A. Rapid amplification of complementary DNA ends for generation of full-length complementary DNAs: thermal RACE. Methods Enzymol. 1993;218:340–356. doi: 10.1016/0076-6879(93)18026-9. [DOI] [PubMed] [Google Scholar]
- Fujio K., Evarts R. P., Hu Z., Marsden E. R., Thorgeirsson S. S. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994 Apr;70(4):511–516. [PubMed] [Google Scholar]
- Germain L., Noël M., Gourdeau H., Marceau N. Promotion of growth and differentiation of rat ductular oval cells in primary culture. Cancer Res. 1988 Jan 15;48(2):368–378. [PubMed] [Google Scholar]
- Grantham J. J. Polycystic kidney disease: hereditary and acquired. Adv Intern Med. 1993;38:409–420. [PubMed] [Google Scholar]
- Grisham J. W. Cell types in rat liver cultures: their identification and isolation. Mol Cell Biochem. 1983;53-54(1-2):23–33. doi: 10.1007/BF00225244. [DOI] [PubMed] [Google Scholar]
- Mauad T. H., van Nieuwkerk C. M., Dingemans K. P., Smit J. J., Schinkel A. H., Notenboom R. G., van den Bergh Weerman M. A., Verkruisen R. P., Groen A. K., Oude Elferink R. P. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994 Nov;145(5):1237–1245. [PMC free article] [PubMed] [Google Scholar]
- Moyer J. H., Lee-Tischler M. J., Kwon H. Y., Schrick J. J., Avner E. D., Sweeney W. E., Godfrey V. L., Cacheiro N. L., Wilkinson J. E., Woychik R. P. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science. 1994 May 27;264(5163):1329–1333. doi: 10.1126/science.8191288. [DOI] [PubMed] [Google Scholar]
- Richards W. G., Yoder B. K., Isfort R. J., Detilleux P. G., Foster C., Neilsen N., Woychik R. P., Wilkinson J. E. Oval cell proliferation associated with the murine insertional mutation TgN737Rpw. Am J Pathol. 1996 Dec;149(6):1919–1930. [PMC free article] [PubMed] [Google Scholar]
- Shiojiri N., Lemire J. M., Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991 May 15;51(10):2611–2620. [PubMed] [Google Scholar]
- Smit J. J., Schinkel A. H., Oude Elferink R. P., Groen A. K., Wagenaar E., van Deemter L., Mol C. A., Ottenhoff R., van der Lugt N. M., van Roon M. A. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993 Nov 5;75(3):451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Wu J. C., Merlino G., Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):674–678. doi: 10.1073/pnas.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder B. K., Richards W. G., Sweeney W. E., Wilkinson J. E., Avener E. D., Woychik R. P. Insertional mutagenesis and molecular analysis of a new gene associated with polycystic kidney disease. Proc Assoc Am Physicians. 1995 Oct;107(3):314–323. [PubMed] [Google Scholar]
- Zhang M., Thorgeirsson S. S. Modulation of connexins during differentiation of oval cells into hepatocytes. Exp Cell Res. 1994 Jul;213(1):37–42. doi: 10.1006/excr.1994.1170. [DOI] [PubMed] [Google Scholar]