Abstract
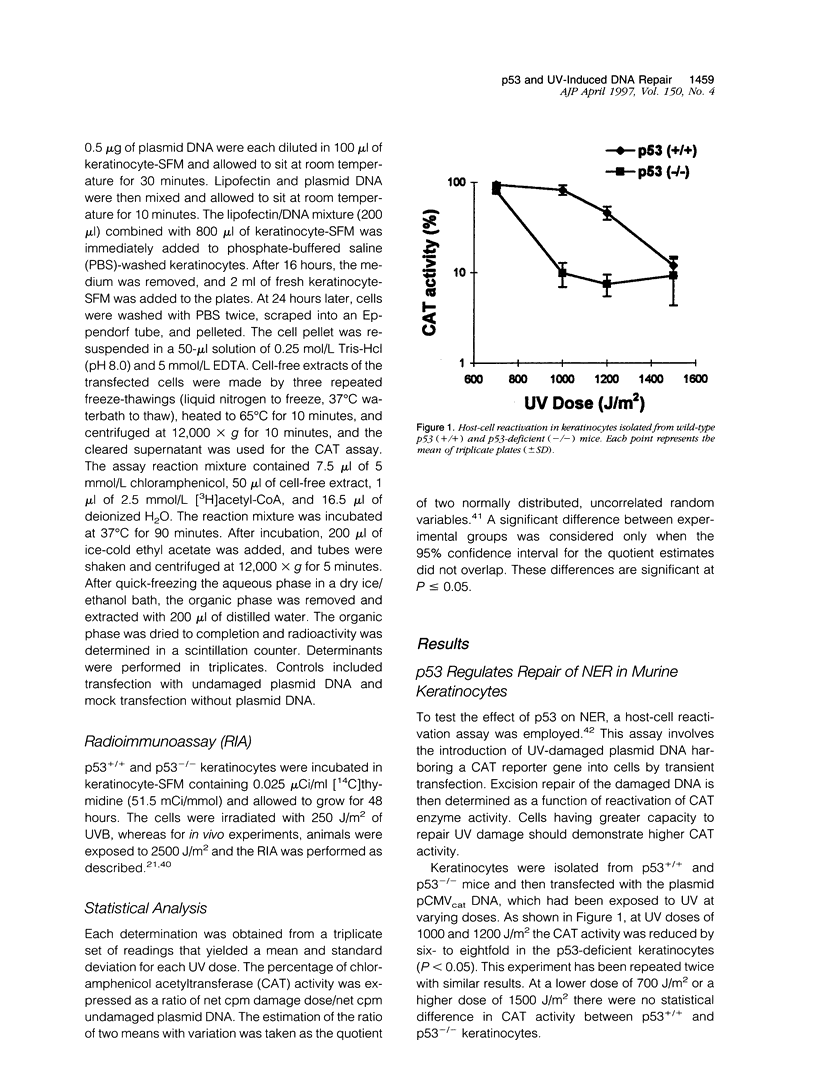
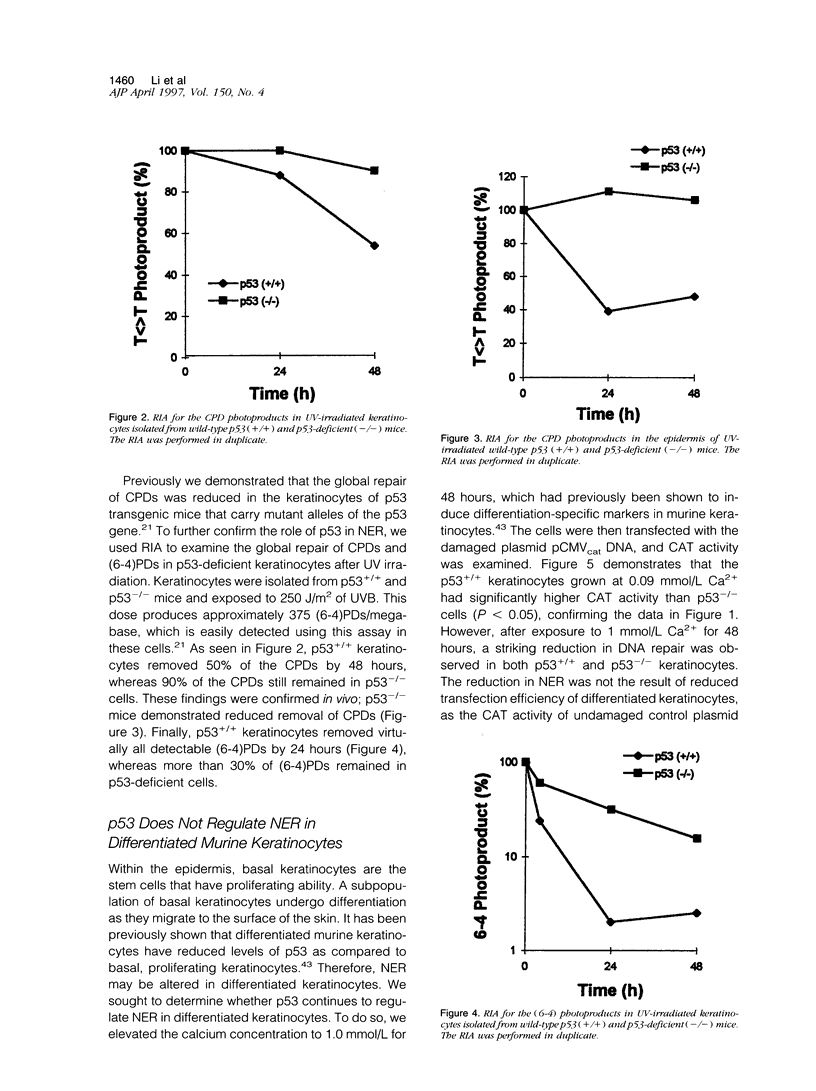
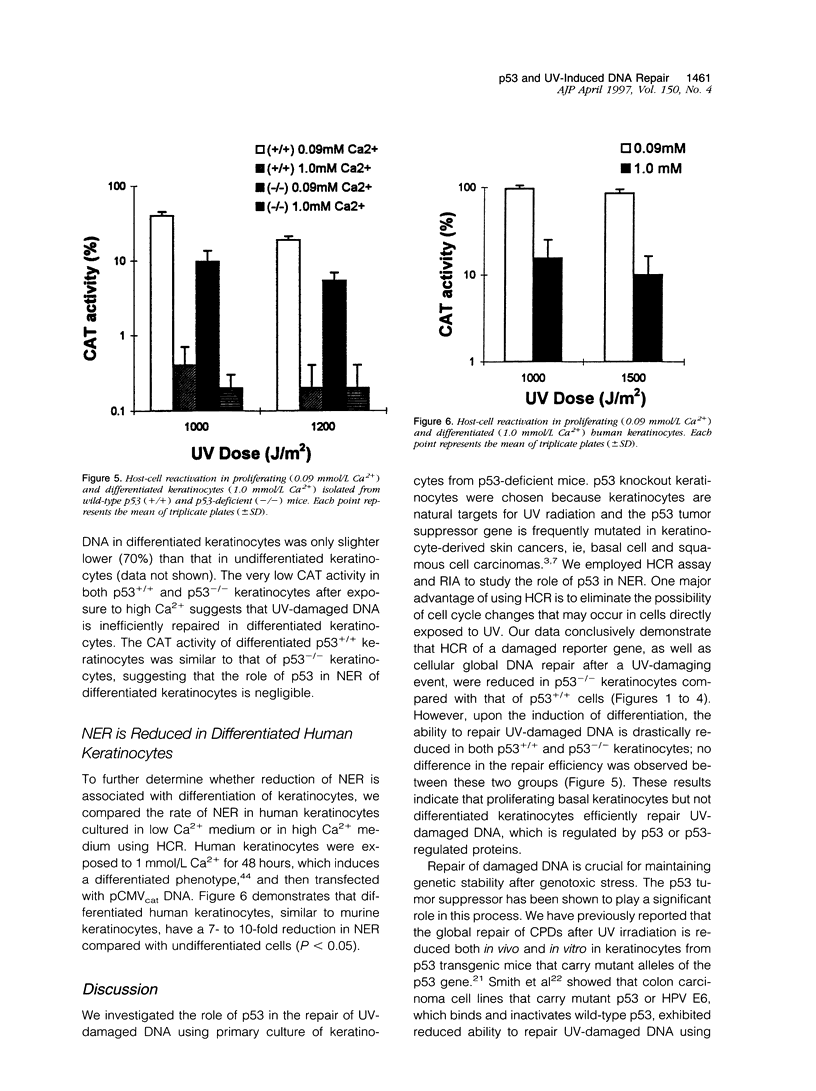
The role of the tumor suppressor p53 in repair of ultraviolet light (UV)-induced DNA damage was evaluated using a host-cell reactivation (HCR) assay. HCR determines a cell's ability to repair UV-damaged DNA through reactivation of a transfected CAT reported plasmid. Most UV damage is removed through nucleotide excision repair (NER). Primary murine keratinocytes isolated from p53-deficient and wild-type p53 mice were used in the HCR assay. The NER was reduced in p53-/- keratinocytes as compared with p53+/+ keratinocytes. The reduced DNA repair in p53-/- mice was confirmed with a radioimmunoassay comparing cyclobutane dimers (CPDs) and (6-4) photoproducts in p53+/+ and p53-/- keratinocytes after the cells were exposed to UV irradiation. Our results demonstrate that wildtype p53 plays a significant role in regulating NER. Furthermore, as there is evidence that p53 protein levels decrease after keratinocytes become differentiated, we sought to determine whether p53 plays a role in NER in differentiated keratinocytes. Differentiation of the keratinocytes by increasing the Ca2+ concentration in the culture media resulted in a marked reduction in NER equally in both p53+/+ and p53-/- groups. This finding suggests that reduced DNA repair after differentiation is p53 independent. A similar reduction in HCR was confirmed in differentiated human keratinocytes. These data, taken together, indicate that p53 or p53-regulated proteins enhance NER in basal undifferentiated keratinocytes but not in differentiated cells. As nonmelanoma skin cancers originate from the basal keratinocytes, our findings suggest that loss of p53 may contribute to the pathogenesis of this common skin cancer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athas W. F., Hedayati M. A., Matanoski G. M., Farmer E. R., Grossman L. Development and field-test validation of an assay for DNA repair in circulating human lymphocytes. Cancer Res. 1991 Nov 1;51(21):5786–5793. [PubMed] [Google Scholar]
- Bakalkin G., Yakovleva T., Selivanova G., Magnusson K. P., Szekely L., Kiseleva E., Klein G., Terenius L., Wiman K. G. p53 binds single-stranded DNA ends and catalyzes DNA renaturation and strand transfer. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):413–417. doi: 10.1073/pnas.91.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T. DNA lesion-recognizing proteins and the p53 connection. Anticancer Res. 1996 Jan-Feb;16(1):225–242. [PubMed] [Google Scholar]
- Boyce S. T., Ham R. G. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983 Jul;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- Burns J. E., Baird M. C., Clark L. J., Burns P. A., Edington K., Chapman C., Mitchell R., Robertson G., Soutar D., Parkinson E. K. Gene mutations and increased levels of p53 protein in human squamous cell carcinomas and their cell lines. Br J Cancer. 1993 Jun;67(6):1274–1284. doi: 10.1038/bjc.1993.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coverley D., Kenny M. K., Lane D. P., Wood R. D. A role for the human single-stranded DNA binding protein HSSB/RPA in an early stage of nucleotide excision repair. Nucleic Acids Res. 1992 Aug 11;20(15):3873–3880. doi: 10.1093/nar/20.15.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Donner E. M., Preston R. J. The relationship between p53 status, DNA repair and chromatid aberration induction in G2 mouse embryo fibroblast cells treated with bleomycin. Carcinogenesis. 1996 May;17(5):1161–1165. doi: 10.1093/carcin/17.5.1161. [DOI] [PubMed] [Google Scholar]
- Dumaz N., Drougard C., Sarasin A., Daya-Grosjean L. Specific UV-induced mutation spectrum in the p53 gene of skin tumors from DNA-repair-deficient xeroderma pigmentosum patients. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10529–10533. doi: 10.1073/pnas.90.22.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J. M., Hanawalt P. C. Li-Fraumeni syndrome fibroblasts homozygous for p53 mutations are deficient in global DNA repair but exhibit normal transcription-coupled repair and enhanced UV resistance. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8876–8880. doi: 10.1073/pnas.92.19.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrest B. A., Soter N. A., Stoff J. S., Mihm M. C., Jr The human sunburn reaction: histologic and biochemical studies. J Am Acad Dermatol. 1981 Oct;5(4):411–422. doi: 10.1016/s0190-9622(81)70103-8. [DOI] [PubMed] [Google Scholar]
- Hainaut P. The tumor suppressor protein p53: a receptor to genotoxic stress that controls cell growth and survival. Curr Opin Oncol. 1995 Jan;7(1):76–82. [PubMed] [Google Scholar]
- Hall P. A., McKee P. H., Menage H. D., Dover R., Lane D. P. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993 Jan;8(1):203–207. [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Harvey M., Vogel H., Morris D., Bradley A., Bernstein A., Donehower L. A. A mutant p53 transgene accelerates tumour development in heterozygous but not nullizygous p53-deficient mice. Nat Genet. 1995 Mar;9(3):305–311. doi: 10.1038/ng0395-305. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Ishizaki K., Ejima Y., Matsunaga T., Hara R., Sakamoto A., Ikenaga M., Ikawa Y., Aizawa S. Increased UV-induced SCEs but normal repair of DNA damage in p53-deficient mouse cells. Int J Cancer. 1994 Jul 15;58(2):254–257. doi: 10.1002/ijc.2910580218. [DOI] [PubMed] [Google Scholar]
- Kanjilal S., Pierceall W. E., Cummings K. K., Kripke M. L., Ananthaswamy H. N. High frequency of p53 mutations in ultraviolet radiation-induced murine skin tumors: evidence for strand bias and tumor heterogeneity. Cancer Res. 1993 Jul 1;53(13):2961–2964. [PubMed] [Google Scholar]
- Kastan M. B., Canman C. E., Leonard C. J. P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 1995 Mar;14(1):3–15. doi: 10.1007/BF00690207. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kress S., Sutter C., Strickland P. T., Mukhtar H., Schweizer J., Schwarz M. Carcinogen-specific mutational pattern in the p53 gene in ultraviolet B radiation-induced squamous cell carcinomas of mouse skin. Cancer Res. 1992 Nov 15;52(22):6400–6403. [PubMed] [Google Scholar]
- Lavigueur A., Maltby V., Mock D., Rossant J., Pawson T., Bernstein A. High incidence of lung, bone, and lymphoid tumors in transgenic mice overexpressing mutant alleles of the p53 oncogene. Mol Cell Biol. 1989 Sep;9(9):3982–3991. doi: 10.1128/mcb.9.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M., Bernstein A. p53 mutations increase resistance to ionizing radiation. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5742–5746. doi: 10.1073/pnas.90.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Li G., Ho V. C., Berean K., Tron V. A. Ultraviolet radiation induction of squamous cell carcinomas in p53 transgenic mice. Cancer Res. 1995 May 15;55(10):2070–2074. [PubMed] [Google Scholar]
- Li G., Mitchell D. L., Ho V. C., Reed J. C., Tron V. A. Decreased DNA repair but normal apoptosis in ultraviolet-irradiated skin of p53-transgenic mice. Am J Pathol. 1996 Apr;148(4):1113–1123. [PMC free article] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Léveillard T., Andera L., Bissonnette N., Schaeffer L., Bracco L., Egly J. M., Wasylyk B. Functional interactions between p53 and the TFIIH complex are affected by tumour-associated mutations. EMBO J. 1996 Apr 1;15(7):1615–1624. [PMC free article] [PubMed] [Google Scholar]
- McGregor J. M., Yu C. C., Dublin E. A., Levison D. A., MacDonald D. M. Aberrant expression of p53 tumour-suppressor protein in non-melanoma skin cancer. Br J Dermatol. 1992 Nov;127(5):463–469. doi: 10.1111/j.1365-2133.1992.tb14841.x. [DOI] [PubMed] [Google Scholar]
- McWhir J., Selfridge J., Harrison D. J., Squires S., Melton D. W. Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning. Nat Genet. 1993 Nov;5(3):217–224. doi: 10.1038/ng1193-217. [DOI] [PubMed] [Google Scholar]
- Mirzayans R., Enns L., Dietrich K., Barley R. D., Paterson M. C. Faulty DNA polymerase delta/epsilon-mediated excision repair in response to gamma radiation or ultraviolet light in p53-deficient fibroblast strains from affected members of a cancer-prone family with Li-Fraumeni syndrome. Carcinogenesis. 1996 Apr;17(4):691–698. doi: 10.1093/carcin/17.4.691. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Harigai M., Hanada M., Reed J. C. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994 Jun 15;54(12):3131–3135. [PubMed] [Google Scholar]
- Miyashita T., Krajewski S., Krajewska M., Wang H. G., Lin H. K., Liebermann D. A., Hoffman B., Reed J. C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994 Jun;9(6):1799–1805. [PubMed] [Google Scholar]
- Rady P., Scinicariello F., Wagner R. F., Jr, Tyring S. K. p53 mutations in basal cell carcinomas. Cancer Res. 1992 Jul 1;52(13):3804–3806. [PubMed] [Google Scholar]
- Sancar A. Mechanisms of DNA excision repair. Science. 1994 Dec 23;266(5193):1954–1956. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- Scherer S. J., Welter C., Zang K. D., Dooley S. Specific in vitro binding of p53 to the promoter region of the human mismatch repair gene hMSH2. Biochem Biophys Res Commun. 1996 Apr 25;221(3):722–728. doi: 10.1006/bbrc.1996.0663. [DOI] [PubMed] [Google Scholar]
- Sim C. S., Slater S., McKee P. H. Mutant p53 expression in solar keratosis: an immunohistochemical study. J Cutan Pathol. 1992 Aug;19(4):302–308. doi: 10.1111/j.1600-0560.1992.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Chen I. T., Zhan Q., O'Connor P. M., Fornace A. J., Jr Involvement of the p53 tumor suppressor in repair of u.v.-type DNA damage. Oncogene. 1995 Mar 16;10(6):1053–1059. [PubMed] [Google Scholar]
- Taguchi M., Watanabe S., Sato Y., Kameya T., Munakata N., Ishihara K., Nakane P. K., Hisatome H., Ikeda S. Immunohistochemical examination of tumor-suppressor gene p53 product and pyrimidine dimer in solar keratosis. J Cancer Res Clin Oncol. 1993;119(5):260–262. doi: 10.1007/BF01212722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. W., Forrester K., Yeh H., Feitelson M. A., Gu J. R., Harris C. C. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. W., Yeh H., Schaeffer L., Roy R., Moncollin V., Egly J. M., Wang Z., Freidberg E. C., Evans M. K., Taffe B. G. p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat Genet. 1995 Jun;10(2):188–195. doi: 10.1038/ng0695-188. [DOI] [PubMed] [Google Scholar]
- Weinberg W. C., Azzoli C. G., Chapman K., Levine A. J., Yuspa S. H. p53-mediated transcriptional activity increases in differentiating epidermal keratinocytes in association with decreased p53 protein. Oncogene. 1995 Jun 15;10(12):2271–2279. [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Sugano T. U.v.-induced nuclear accumulation of p53 is evoked through DNA damage of actively transcribed genes independent of the cell cycle. Oncogene. 1994 Oct;9(10):2775–2784. [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Jonason A. S., Leffell D. J., Simon J. A., Sharma H. W., Kimmelman J., Remington L., Jacks T., Brash D. E. Sunburn and p53 in the onset of skin cancer. Nature. 1994 Dec 22;372(6508):773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]


