Abstract
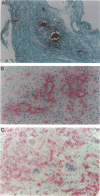
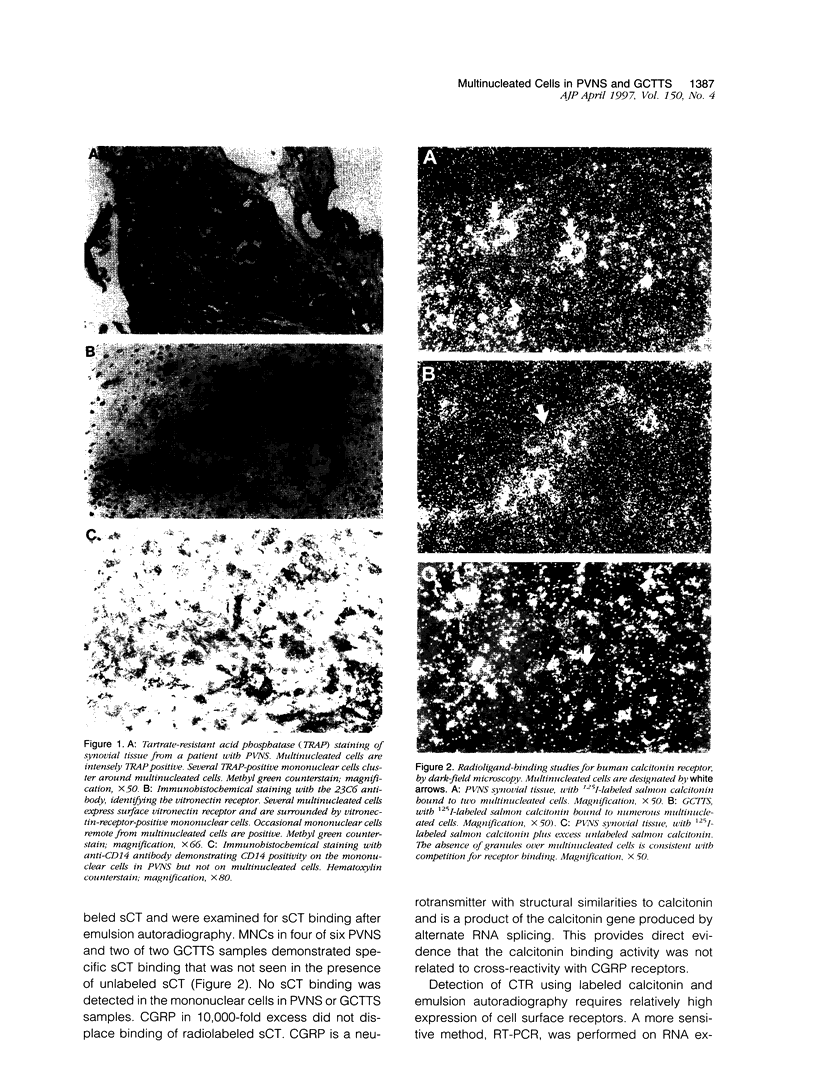
Pigmented villonodular synovitis (PVNS) and the histologically related lesion giant cell tumor of tendon sheath (GCTTS) are idiopathic, proliferative lesions that can induce osteolysis and formation of bone cysts. These lesions contain two predominant cell types: mononuclear polyhedral cells and multinucleated cells (MNCs). Previous studies demonstrated that the mononuclear cells exhibit phenotypic features consistent with derivation from a monocyte/macrophage lineage. The cell lineage of the MNCs and their relationship to osteoclasts are not known. To characterize the MNCs in these lesions and to establish the relationship of these MNCs to osteoclasts, histological sections from six cases of PVNS and two cases of GCTTS were studied. Mononuclear cells expressed CD14 and HLA-DR, in keeping with their relationship to cells of the monocyte/macrophage lineage. Characterization of the MNCs revealed features associated with an osteoclast phenotype. Seven of the eight specimens contained MNCs that were intensely tartrate-resistant acid phosphatase positive; approximately 5% of the mononuclear cells were tartrate-resistant acid phosphatase positive, and these tended to surround MNCs. MNCs in both lesions reacted strongly with the 23C6 monoclonal antibody that recognizes the alpha V beta 3 integrin (the vitronectin receptor), as did several mononuclear cells surrounding the MNCs. Most MNCs did not express CD14 or HLA-DR. Expression of receptors for calcitonin, a marker for osteoclasts, was detected on MNCs after incubation of sections with 125I-labeled salmon calcitonin and emulsion autoradiography. MNCs in four of six PVNS and two of two GCTTS samples demonstrated specific calcitonin binding. Expression of mRNA for calcitonin receptor was confirmed in all cases by reverse transcriptase polymerase chain reaction. These results demonstrate that MNCs in PVNS and GCTTS express phenotypic features of authentic osteoclasts and suggest that osteoclast-like multinucleated cells can arise in synovial soft tissues remote from bone.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alguacil-Garcia A., Unni K. K., Goellner J. R. Giant cell tumor of tendon sheath and pigmented villonodular synovitis: an ultrastructural study. Am J Clin Pathol. 1978 Jan;69(1):6–17. doi: 10.1093/ajcp/69.1.6. [DOI] [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J., Ferguson D. J., McGee J. O. Bone resorption by macrophage polykaryons of giant cell tumour of tendon sheath. Br J Cancer. 1991 Apr;63(4):527–533. doi: 10.1038/bjc.1991.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J., Horton M. A., McGee J. O. New sites of cellular vitronectin receptor immunoreactivity detected with osteoclast-reacting monoclonal antibodies 13C2 and 23C6. Bone Miner. 1990 Jan;8(1):7–22. doi: 10.1016/0169-6009(91)90136-n. [DOI] [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J. Immunophenotypic differences between osteoclasts and macrophage polykaryons: immunohistological distinction and implications for osteoclast ontogeny and function. J Clin Pathol. 1990 Dec;43(12):997–1003. doi: 10.1136/jcp.43.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P., Costantini M., Dearden L. C., Bonucci E. Expression of tartrate-resistant acid phosphatase in bone marrow macrophages. Basic Appl Histochem. 1987;31(4):433–440. [PubMed] [Google Scholar]
- Byers P. D., Cotton R. E., Deacon O. W., Lowy M., Newman P. H., Sissons H. A., Thomson A. D. The diagnosis and treatment of pigmented villonodular synovitis. J Bone Joint Surg Br. 1968 May;50(2):290–305. [PubMed] [Google Scholar]
- CHUNG S. M., JANES J. M. DIFFUSE PIGMENTED VILLONODULAR SYNOVITIS OF THE HIP JOINT. REVIEW OF THE LITERATURE AND REPORT OF FOUR CASES. J Bone Joint Surg Am. 1965 Mar;47:293–303. [PubMed] [Google Scholar]
- Carstens H. B. Giant cell tumors of tendon sheath. An electron microscopical study of 11 cases. Arch Pathol Lab Med. 1978 Feb;102(2):99–103. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Connor J. R., Dodds R. A., James I. E., Gowen M. Human osteoclast and giant cell differentiation: the apparent switch from nonspecific esterase to tartrate resistant acid phosphatase activity coincides with the in situ expression of osteopontin mRNA. J Histochem Cytochem. 1995 Dec;43(12):1193–1201. doi: 10.1177/43.12.8537635. [DOI] [PubMed] [Google Scholar]
- Darling J. M., Glimcher L. H., Shortkroff S., Albano B., Gravallese E. M. Expression of metalloproteinases in pigmented villonodular synovitis. Hum Pathol. 1994 Aug;25(8):825–830. doi: 10.1016/0046-8177(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Dorwart R. H., Genant H. K., Johnston W. H., Morris J. M. Pigmented villonodular synovitis of synovial joints: clinical, pathologic, and radiologic features. AJR Am J Roentgenol. 1984 Oct;143(4):877–885. doi: 10.2214/ajr.143.4.877. [DOI] [PubMed] [Google Scholar]
- Eisenstein R. Giant-cell tumor of tendon sheath. Its histogenesis as studied in the electron microscope. J Bone Joint Surg Am. 1968 Apr;50(3):476–486. doi: 10.2106/00004623-196850030-00006. [DOI] [PubMed] [Google Scholar]
- Esmaili J. H., Hafez G. R., Warner T. F. Anaplastic carcinoma of the thyroid with osteoclast-like giant cells. Cancer. 1983 Dec 1;52(11):2122–2128. doi: 10.1002/1097-0142(19831201)52:11<2122::aid-cncr2820521125>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gehweiler J. A., Wilson J. W. Diffuse biarticular pigmented villonodular synovitis. Radiology. 1969 Oct;93(4):845–851. doi: 10.1148/93.4.845. [DOI] [PubMed] [Google Scholar]
- Gitelis S., Heligman D., Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989 Feb;(239):154–160. [PubMed] [Google Scholar]
- Glowacki J., Rey C., Glimcher M. J., Cox K. A., Lian J. A role for osteocalcin in osteoclast differentiation. J Cell Biochem. 1991 Mar;45(3):292–302. doi: 10.1002/jcb.240450312. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Roelke M. S., Petrison K. K., Bhan A. K. Human giant cell tumors of bone identification and characterization of cell types. J Clin Invest. 1987 Feb;79(2):483–491. doi: 10.1172/JCI112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring S. R., Schiller A. L., Mankin H. J., Dayer J. M., Krane S. M. Characterization of cells from human giant cell tumors of bone. Clin Orthop Relat Res. 1986 Mar;(204):59–75. [PubMed] [Google Scholar]
- Gorn A. H., Lin H. Y., Yamin M., Auron P. E., Flannery M. R., Tapp D. R., Manning C. A., Lodish H. F., Krane S. M., Goldring S. R. Cloning, characterization, and expression of a human calcitonin receptor from an ovarian carcinoma cell line. J Clin Invest. 1992 Nov;90(5):1726–1735. doi: 10.1172/JCI116046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese E. M., Darling J. M., Ladd A. L., Katz J. N., Glimcher L. H. In situ hybridization studies of stromelysin and collagenase messenger RNA expression in rheumatoid synovium. Arthritis Rheum. 1991 Sep;34(9):1076–1084. doi: 10.1002/art.1780340903. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology. 1989 Sep;125(3):1606–1612. doi: 10.1210/endo-125-3-1606. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Generation of osteoclastic function in mouse bone marrow cultures: multinuclearity and tartrate-resistant acid phosphatase are unreliable markers for osteoclastic differentiation. Endocrinology. 1989 Apr;124(4):1689–1696. doi: 10.1210/endo-124-4-1689. [DOI] [PubMed] [Google Scholar]
- Holtrop M. E., Raisz L. G., Simmons H. A. The effects of parathyroid hormone, colchicine, and calcitonin on the ultrastructure and the activity of osteoclasts in organ culture. J Cell Biol. 1974 Feb;60(2):346–355. doi: 10.1083/jcb.60.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- Horton M. Vitronectin receptor: tissue specific expression or adaptation to culture? Int J Exp Pathol. 1990 Oct;71(5):741–759. [PMC free article] [PubMed] [Google Scholar]
- Ikegame M., Rakopoulos M., Zhou H., Houssami S., Martin T. J., Moseley J. M., Findlay D. M. Calcitonin receptor isoforms in mouse and rat osteoclasts. J Bone Miner Res. 1995 Jan;10(1):59–65. doi: 10.1002/jbmr.5650100110. [DOI] [PubMed] [Google Scholar]
- Józsa L. Immunohistochemical characterization of pigmented villonodular synovitis. Zentralbl Pathol. 1992 Apr;138(2):119–123. [PubMed] [Google Scholar]
- Lee S. K., Goldring S. R., Lorenzo J. A. Expression of the calcitonin receptor in bone marrow cell cultures and in bone: a specific marker of the differentiated osteoclast that is regulated by calcitonin. Endocrinology. 1995 Oct;136(10):4572–4581. doi: 10.1210/endo.136.10.7664679. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Harris T. L., Flannery M. S., Aruffo A., Kaji E. H., Gorn A., Kolakowski L. F., Jr, Lodish H. F., Goldring S. R. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science. 1991 Nov 15;254(5034):1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- Ling L., Klein M. J., Sissons H. A., Steiner G. C. Lysozyme and alpha 1-antitrypsin in giant-cell tumor of bone and in other lesions that contain giant cells. Arch Pathol Lab Med. 1986 Aug;110(8):713–718. [PubMed] [Google Scholar]
- Ling L., Klein M. J., Sissons H. A., Steiner G. C., Winchester R. J. Expression of Ia and monocyte-macrophage lineage antigens in giant cell tumor of bone and related lesions. Arch Pathol Lab Med. 1988 Jan;112(1):65–69. [PubMed] [Google Scholar]
- Myers B. W., Masi A. T. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine (Baltimore) 1980 May;59(3):223–238. [PubMed] [Google Scholar]
- Nielsen A. L., Kiaer T. Malignant giant cell tumor of synovium and locally destructive pigmented villonodular synovitis: ultrastructural and immunohistochemical study and review of the literature. Hum Pathol. 1989 Aug;20(8):765–771. doi: 10.1016/0046-8177(89)90070-1. [DOI] [PubMed] [Google Scholar]
- O'Connell J. X., Fanburg J. C., Rosenberg A. E. Giant cell tumor of tendon sheath and pigmented villonodular synovitis: immunophenotype suggests a synovial cell origin. Hum Pathol. 1995 Jul;26(7):771–775. doi: 10.1016/0046-8177(95)90226-0. [DOI] [PubMed] [Google Scholar]
- Ono S. J., Bazil V., Sugawara M., Strominger J. L. An isotype-specific trans-acting factor is defective in a mutant B cell line that expresses HLA-DQ, but not -DR or -DP. J Exp Med. 1991 Mar 1;173(3):629–637. doi: 10.1084/jem.173.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinini L. A., Goldsmith N. K., Schachter B. S., Davies T. F. Localization of HLA-DR alpha-chain messenger ribonucleic acid in normal and autoimmune human thyroid using in situ hybridization. J Clin Endocrinol Metab. 1988 Jun;66(6):1307–1315. doi: 10.1210/jcem-66-6-1307. [DOI] [PubMed] [Google Scholar]
- Reginato A., Martinez V., Schumacher H. R., Torres J. Giant cell tumour associated with rheumatoid arthritis. Ann Rheum Dis. 1974 Jul;33(4):333–341. doi: 10.1136/ard.33.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J. H., PUGH D. G. Roentgenographic aspects of articular pigmented villonodular synovitis. Am J Roentgenol Radium Ther Nucl Med. 1962 Jun;87:1146–1156. [PubMed] [Google Scholar]
- Schumacher H. R., Lotke P., Athreya B., Rothfuss S. Pigmented villonodular synovitis: light and electron microscopic studies. Semin Arthritis Rheum. 1982 Aug;12(1):32–43. doi: 10.1016/0049-0172(82)90021-x. [DOI] [PubMed] [Google Scholar]
- Scott P. M. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968 May;50(2):306–311. [PubMed] [Google Scholar]
- Suda T., Takahashi N., Martin T. J. Modulation of osteoclast differentiation. Endocr Rev. 1992 Feb;13(1):66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Akatsu T., Sasaki T., Nicholson G. C., Moseley J. M., Martin T. J., Suda T. Induction of calcitonin receptors by 1 alpha, 25-dihydroxyvitamin D3 in osteoclast-like multinucleated cells formed from mouse bone marrow cells. Endocrinology. 1988 Sep;123(3):1504–1510. doi: 10.1210/endo-123-3-1504. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Goldring S., Katz M., Hilsenbeck S., Williams R., Roodman G. D. Downregulation of calcitonin receptor mRNA expression by calcitonin during human osteoclast-like cell differentiation. J Clin Invest. 1995 Jan;95(1):167–171. doi: 10.1172/JCI117634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro H., Iwasaki H., Kikuchi M., Ogata K., Okazaki M. Giant cell tumors of tendon sheath: a single and multiple immunostaining analysis. Pathol Int. 1995 Feb;45(2):147–155. doi: 10.1111/j.1440-1827.1995.tb03435.x. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Beckstead J. H., Medeiros L. J., Kempson R. L., Warnke R. A. The cells of giant cell tumor of tendon sheath resemble osteoclasts. Am J Surg Pathol. 1988 Jun;12(6):444–452. doi: 10.1097/00000478-198806000-00004. [DOI] [PubMed] [Google Scholar]
- van de Wijngaert F. P., Burger E. H. Demonstration of tartrate-resistant acid phosphatase in un-decalcified, glycolmethacrylate-embedded mouse bone: a possible marker for (pre)osteoclast identification. J Histochem Cytochem. 1986 Oct;34(10):1317–1323. doi: 10.1177/34.10.3745910. [DOI] [PubMed] [Google Scholar]