Abstract
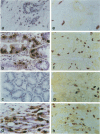
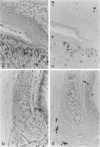
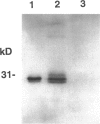
Soluble granule chymases in rodent intestinal mucosal mast cells (IMMCs) may play an important role in altering epithelial permeability during immediate hypersensitivity reactions. Using a monoclonal antibody against the chymase mouse mast cell protease-1 (mMCP-1), we have shown that it is constitutively expressed in < or = 20% of esterase-positive (esterase+) IMMCs but not in esterase+ gastric mucosal mast cells (GMMCs) in normal BALB/c mice. Intestinal infection with mouse- or rat-adapted strains of Nippostrongylus brasiliensis resulted in IMMC hyperplasia with 100% of esterase+ IMMCs expressing mMCP-1. In contrast, there was a variable response in terms of numbers of GMMCs and of the proportion expressing mMCP-1. Esterase+ mast cells in the gastric submucosa, muscularis, ear pinna, lung parenchyma, major airway submucosa, and peritoneal cavity did not express mMCP-1. The few airway esterase+ mast cells expressing mMCP-1 were, like the great majority of IMMCs and GMMCs, located intraepithelially. In conclusion, mMCP-1 is predominantly expressed by intraepithelial mucosal mast cells but not in all sites; the immunological stimulus associated with intestinal nematodiasis substantially up-regulates mMCP-1 expression by mast cells in the jejunum but not in the stomach; IMMCs and GMMCs in BALB/c mice are phenotypically and possibly functionally distinct.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldenborg F., Enerbäck L. The immunohistochemical demonstration of chymase and tryptase in human intestinal mast cells. Histochem J. 1994 Jul;26(7):587–596. doi: 10.1007/BF00158593. [DOI] [PubMed] [Google Scholar]
- Catto-Smith A. G., Ripper J. L. Mucosal mast cells and developmental changes in gastric absorption. Am J Physiol. 1995 Jan;268(1 Pt 1):G121–G127. doi: 10.1152/ajpgi.1995.268.1.G121. [DOI] [PubMed] [Google Scholar]
- Crowe S. E., Perdue M. H. Gastrointestinal food hypersensitivity: basic mechanisms of pathophysiology. Gastroenterology. 1992 Sep;103(3):1075–1095. doi: 10.1016/0016-5085(92)90047-3. [DOI] [PubMed] [Google Scholar]
- Du T., Friend D. S., Austen K. F., Katz H. R. Tissue-dependent differences in the asynchronous appearance of mast cells in normal mice and in congenic mast cell-deficient mice after infusion of normal bone marrow cells. Clin Exp Immunol. 1996 Feb;103(2):316–321. doi: 10.1046/j.1365-2249.1996.d01-610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. S., Ghildyal N., Austen K. F., Gurish M. F., Matsumoto R., Stevens R. L. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996 Oct;135(1):279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J. New insights into "the riddle of the mast cells": microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990 Jan;62(1):5–33. [PubMed] [Google Scholar]
- Ghildyal N., Friend D. S., Nicodemus C. F., Austen K. F., Stevens R. L. Reversible expression of mouse mast cell protease 2 mRNA and protein in cultured mast cells exposed to IL-10. J Immunol. 1993 Sep 15;151(6):3206–3214. [PubMed] [Google Scholar]
- Ghildyal N., McNeil H. P., Stechschulte S., Austen K. F., Silberstein D., Gurish M. F., Somerville L. L., Stevens R. L. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J Immunol. 1992 Sep 15;149(6):2123–2129. [PubMed] [Google Scholar]
- Gurish M. F., Pear W. S., Stevens R. L., Scott M. L., Sokol K., Ghildyal N., Webster M. J., Hu X., Austen K. F., Baltimore D. Tissue-regulated differentiation and maturation of a v-abl-immortalized mast cell-committed progenitor. Immunity. 1995 Aug;3(2):175–186. doi: 10.1016/1074-7613(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Huntley J. F., Gooden C., Newlands G. F., Mackellar A., Lammas D. A., Wakelin D., Tuohy M., Woodbury R. G., Miller H. R. Distribution of intestinal mast cell proteinase in blood and tissues of normal and Trichinella-infected mice. Parasite Immunol. 1990 Jan;12(1):85–95. doi: 10.1111/j.1365-3024.1990.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Huntley J. F., Newlands G. F., Gibson S., Ferguson A., Miller H. R. Histochemical demonstration of chymotrypsin like serine esterases in mucosal mast cells in four species including man. J Clin Pathol. 1985 Apr;38(4):375–384. doi: 10.1136/jcp.38.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Kitamura Y., Shimada M., Hatanaka K., Miyano Y. Development of mast cells from grafted bone marrow cells in irradiated mice. Nature. 1977 Aug 4;268(5619):442–443. doi: 10.1038/268442a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Nakano T., Nakahata T., Asai H., Yagi Y., Tsuji K., Komiyama A., Akabane T., Kojima S., Kitamura Y. Formation of mast cell colonies in methylcellulose by mouse peritoneal cells and differentiation of these cloned cells in both the skin and the gastric mucosa of W/Wv mice: evidence that a common precursor can give rise to both "connective tissue-type" and "mucosal" mast cells. J Immunol. 1986 Feb 15;136(4):1378–1384. [PubMed] [Google Scholar]
- Miller H. R., Huntley J. F., Newlands G. F., Mackellar A., Lammas D. A., Wakelin D. Granule proteinases define mast cell heterogeneity in the serosa and the gastrointestinal mucosa of the mouse. Immunology. 1988 Dec;65(4):559–566. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Jarrett W. F. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology. 1971 Mar;20(3):277–288. [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985 Sep 1;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa Y., Miller H. R. Protection against Nippostrongylus brasiliensis by adoptive immunization with immune thoracic duct lymphocytes. Cell Immunol. 1978 Apr;37(1):51–60. doi: 10.1016/0008-8749(78)90173-9. [DOI] [PubMed] [Google Scholar]
- Newlands G. F., Gibson S., Knox D. P., Grencis R., Wakelin D., Miller H. R. Characterization and mast cell origin of a chymotrypsin-like proteinase isolated from intestines of mice infected with Trichinella spiralis. Immunology. 1987 Dec;62(4):629–634. [PMC free article] [PubMed] [Google Scholar]
- Newlands G. F., Huntley J. F., Miller H. R. Concomitant detection of mucosal mast cells and eosinophils in the intestines of normal and Nippostrongylus-immune rats. A re-evaluation of histochemical and immunocytochemical techniques. Histochemistry. 1984;81(6):585–589. doi: 10.1007/BF00489539. [DOI] [PubMed] [Google Scholar]
- Newlands G. F., Knox D. P., Pirie-Shepherd S. R., Miller H. R. Biochemical and immunological characterization of multiple glycoforms of mouse mast cell protease 1: comparison with an isolated murine serosal mast cell protease (MMCP-4). Biochem J. 1993 Aug 15;294(Pt 1):127–135. doi: 10.1042/bj2940127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald H. R., Dessing M., Dvorak A. M., Galli S. J. Identification of a committed precursor for the mast cell lineage. Science. 1996 Feb 9;271(5250):818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- Scudamore C. L., Pennington A. M., Thornton E., McMillan L., Newlands G. F., Miller H. R. Basal secretion and anaphylactic release of rat mast cell protease-II (RMCP-II) from ex vivo perfused rat jejunum: translocation of RMCP-II into the gut lumen and its relation to mucosal histology. Gut. 1995 Aug;37(2):235–241. doi: 10.1136/gut.37.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudamore C. L., Thornton E. M., McMillan L., Newlands G. F., Miller H. R. Release of the mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J Exp Med. 1995 Dec 1;182(6):1871–1881. doi: 10.1084/jem.182.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä H. E. Rat skin main neutral protease: immunohistochemical localization. J Invest Dermatol. 1978 Nov;71(5):311–315. doi: 10.1111/1523-1747.ep12529791. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Weis J. H. Mucosal T cells and mast cells share common adhesion receptors. Immunol Today. 1996 Feb;17(2):60–63. doi: 10.1016/0167-5699(96)80580-9. [DOI] [PubMed] [Google Scholar]
- Sonoda S., Sonoda T., Nakano T., Kanayama Y., Kanakura Y., Asai H., Yonezawa T., Kitamura Y. Development of mucosal mast cells after injection of a single connective tissue-type mast cell in the stomach mucosa of genetically mast cell-deficient W/Wv mice. J Immunol. 1986 Aug 15;137(4):1319–1322. [PubMed] [Google Scholar]
- Stevens R. L., Friend D. S., McNeil H. P., Schiller V., Ghildyal N., Austen K. F. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):128–132. doi: 10.1073/pnas.91.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trong H. L., Newlands G. F., Miller H. R., Charbonneau H., Neurath H., Woodbury R. G. Amino acid sequence of a mouse mucosal mast cell protease. Biochemistry. 1989 Jan 10;28(1):391–395. doi: 10.1021/bi00427a054. [DOI] [PubMed] [Google Scholar]
- Wershil B. K., Furuta G. T., Wang Z. S., Galli S. J. Mast cell-dependent neutrophil and mononuclear cell recruitment in immunoglobulin E-induced gastric reactions in mice. Gastroenterology. 1996 May;110(5):1482–1490. doi: 10.1053/gast.1996.v110.pm8613053. [DOI] [PubMed] [Google Scholar]
- Wong D., Ogle C. W. Chronic parenterally administered nicotine and stress- or ethanol-induced gastric mucosal damage in rats. Eur J Pharmacol. 1995 Jan 13;292(2):157–162. doi: 10.1016/0926-6917(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R., Huntley J. F., Newlands G. F., Palliser A. C., Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. 1984 Nov 29-Dec 5Nature. 312(5993):450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- Xia Z., Ghildyal N., Austen K. F., Stevens R. L. Post-transcriptional regulation of chymase expression in mast cells. A cytokine-dependent mechanism for controlling the expression of granule neutral proteases of hematopoietic cells. J Biol Chem. 1996 Apr 12;271(15):8747–8753. doi: 10.1074/jbc.271.15.8747. [DOI] [PubMed] [Google Scholar]