Abstract
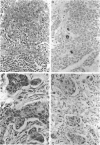
Clinicopathological evidence has accumulated that colorectal adenocarcinoma with minimal or no glandular differentiation constitutes two entities with different prognosis. In a series of 20 predominantly nonglandular, poorly differentiated adenocarcinomas, histological features, DNA content, p53 protein expression, Ki-ras mutation, and microsatellite instability were analyzed and correlated to the biology of the tumors. In addition, the presence of Epstein-Barr virus (EBV) transcripts was tested by RNA in situ hybridization and EBV DNA was demonstrated by nested polymerase chain reaction. Histologically, 13 tumors showed small uniform cells and 7 tumors showed large pleomorphic cells. Tumors with uniform cells exhibited more commonly an expansive growth pattern (69.2% versus 0%; P < 0.025) and a dense peritumor lymphoid infiltrate (84.6% versus 14.3%; P < 0.01) resembling their gastric counterpart, solid or medullary carcinoma. These tumors showed less frequent lymph node as well as hematogeneous metastases than pleomorphic carcinomas. In addition, they were usually diploid (84.6% versus 28.6%; P < 0.05) and lacked stabilization of the p53 protein (0% versus 42.9%; P < 0.05). No significant difference between the medullary and the pleomorphic tumor type was found with respect to bcl2 expression and the occurrence of Ki-ras mutations at codon 12. In contrast, microsatellite instability was almost totally restricted to poorly differentiated adenocarcinomas of the medullary type (100% versus 14.3%; P < 0.001). Finally, polymerase chain reaction revealed EBV DNA in 5 tumor specimens, which was, however, restricted to the peritumor lymphoid infiltrate as shown by in situ hybridization. Correlation with the biology of the tumors revealed that only one patient with the uniform cell type died due to metastastic disease during the follow-up period (median, 31 months), which was the case in five of the seven patients with the pleomorphic-type carcinoma (P < 0.025). Our results clearly indicate that the poorly differentiated colonic carcinoma with minimal or no glandular structures constitute two different entities, a medullary and a pleomorphic variant, which markedly differ in their phenotype, genotype, and prognosis.
Full text
PDF









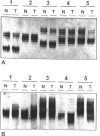
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Ambinder R. F., Mann R. B. Detection and characterization of Epstein-Barr virus in clinical specimens. Am J Pathol. 1994 Aug;145(2):239–252. [PMC free article] [PubMed] [Google Scholar]
- Baas I. O., Mulder J. W., Offerhaus G. J., Vogelstein B., Hamilton S. R. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994 Jan;172(1):5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- Bocker T., Schlegel J., Kullmann F., Stumm G., Zirngibl H., Epplen J. T., Rüschoff J. Genomic instability in colorectal carcinomas: comparison of different evaluation methods and their biological significance. J Pathol. 1996 May;179(1):15–19. doi: 10.1002/(SICI)1096-9896(199605)179:1<15::AID-PATH553>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Branch P., Bicknell D. C., Rowan A., Bodmer W. F., Karran P. Immune surveillance in colorectal carcinoma. Nat Genet. 1995 Mar;9(3):231–232. doi: 10.1038/ng0395-231. [DOI] [PubMed] [Google Scholar]
- Chong J. M., Fukayama M., Hayashi Y., Takizawa T., Koike M., Konishi M., Kikuchi-Yanoshita R., Miyaki M. Microsatellite instability in the progression of gastric carcinoma. Cancer Res. 1994 Sep 1;54(17):4595–4597. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Fukayama M., Hayashi Y., Iwasaki Y., Chong J., Ooba T., Takizawa T., Koike M., Mizutani S., Miyaki M., Hirai K. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab Invest. 1994 Jul;71(1):73–81. [PubMed] [Google Scholar]
- Gibbs N. M. Undifferentiated carcinoma of the large intestine. Histopathology. 1977 Jan;1(1):77–84. doi: 10.1111/j.1365-2559.1977.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Graham D. M., Appelman H. D. Crohn's-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Mod Pathol. 1990 May;3(3):332–335. [PubMed] [Google Scholar]
- Jass J. R. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986 Jun;39(6):585–589. doi: 10.1136/jcp.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jass J. R., Smyrk T. C., Stewart S. M., Lane M. R., Lanspa S. J., Lynch H. T. Pathology of hereditary non-polyposis colorectal cancer. Anticancer Res. 1994 Jul-Aug;14(4B):1631–1634. [PubMed] [Google Scholar]
- Kim H., Jen J., Vogelstein B., Hamilton S. R. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994 Jul;145(1):148–156. [PMC free article] [PubMed] [Google Scholar]
- Lanspa S. J., Lynch H. T., Smyrk T. C., Strayhorn P., Watson P., Lynch J. F., Jenkins J. X., Appelman H. D. Colorectal adenomas in the Lynch syndromes. Results of a colonoscopy screening program. Gastroenterology. 1990 May;98(5 Pt 1):1117–1122. doi: 10.1016/0016-5085(90)90323-s. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Peltomäki P., Meling G. I., Aaltonen L. A., Nyström-Lahti M., Pylkkänen L., Heimdal K., Andersen T. I., Møller P., Rognum T. O. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993 Dec 15;53(24):5849–5852. [PubMed] [Google Scholar]
- Lothe R. A., Peltomäki P., Meling G. I., Aaltonen L. A., Nyström-Lahti M., Pylkkänen L., Heimdal K., Andersen T. I., Møller P., Rognum T. O. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993 Dec 15;53(24):5849–5852. [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T., McGinn T., Lanspa S., Cavalieri J., Lynch J., Slominski-Castor S., Cayouette M. C., Priluck I., Luce M. C. Attenuated familial adenomatous polyposis (AFAP). A phenotypically and genotypically distinctive variant of FAP. Cancer. 1995 Dec 15;76(12):2427–2433. doi: 10.1002/1097-0142(19951215)76:12<2427::aid-cncr2820761205>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Minamoto T., Sawaguchi K., Ohta T., Itoh T., Mai M. Superficial-type adenomas and adenocarcinomas of the colon and rectum: a comparative morphological study. Gastroenterology. 1994 Jun;106(6):1436–1443. doi: 10.1016/0016-5085(94)90395-6. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Ueki T., Yao T., Ueyama T., Tsuneyoshi M. Epstein-Barr virus in gastric carcinoma with lymphoid stroma. Special reference to its detection by the polymerase chain reaction and in situ hybridization in 99 tumors, including a morphologic analysis. Cancer. 1994 May 1;73(9):2239–2249. doi: 10.1002/1097-0142(19940501)73:9<2239::aid-cncr2820730902>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rüschoff J., Bocker T., Schlegel J., Stumm G., Hofstaedter F. Microsatellite instability: new aspects in the carcinogenesis of colorectal carcinoma. Virchows Arch. 1995;426(3):215–222. doi: 10.1007/BF00191357. [DOI] [PubMed] [Google Scholar]
- Schlegel J., Bocker T., Zirngibl H., Hofstädter F., Rüschoff J. Detection of microsatellite instability in human colorectal carcinomas using a non-radioactive PCR-based screening technique. Virchows Arch. 1995;426(3):223–227. doi: 10.1007/BF00191358. [DOI] [PubMed] [Google Scholar]
- Schlegel J., Stumm G., Scherthan H., Bocker T., Zirngibl H., Rüschoff J., Hofstädter F. Comparative genomic in situ hybridization of colon carcinomas with replication error. Cancer Res. 1995 Dec 15;55(24):6002–6005. [PubMed] [Google Scholar]
- Vasen H. F., Nagengast F. M., Khan P. M. Interval cancers in hereditary non-polyposis colorectal cancer (Lynch syndrome) Lancet. 1995 May 6;345(8958):1183–1184. doi: 10.1016/s0140-6736(95)91016-6. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Muto T., Sawada T., Miyaki M. Flat adenoma as a precursor of colorectal carcinoma in hereditary nonpolyposis colorectal carcinoma. Cancer. 1996 Feb 15;77(4):627–634. doi: 10.1002/(sici)1097-0142(19960215)77:4<627::aid-cncr7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]