Abstract
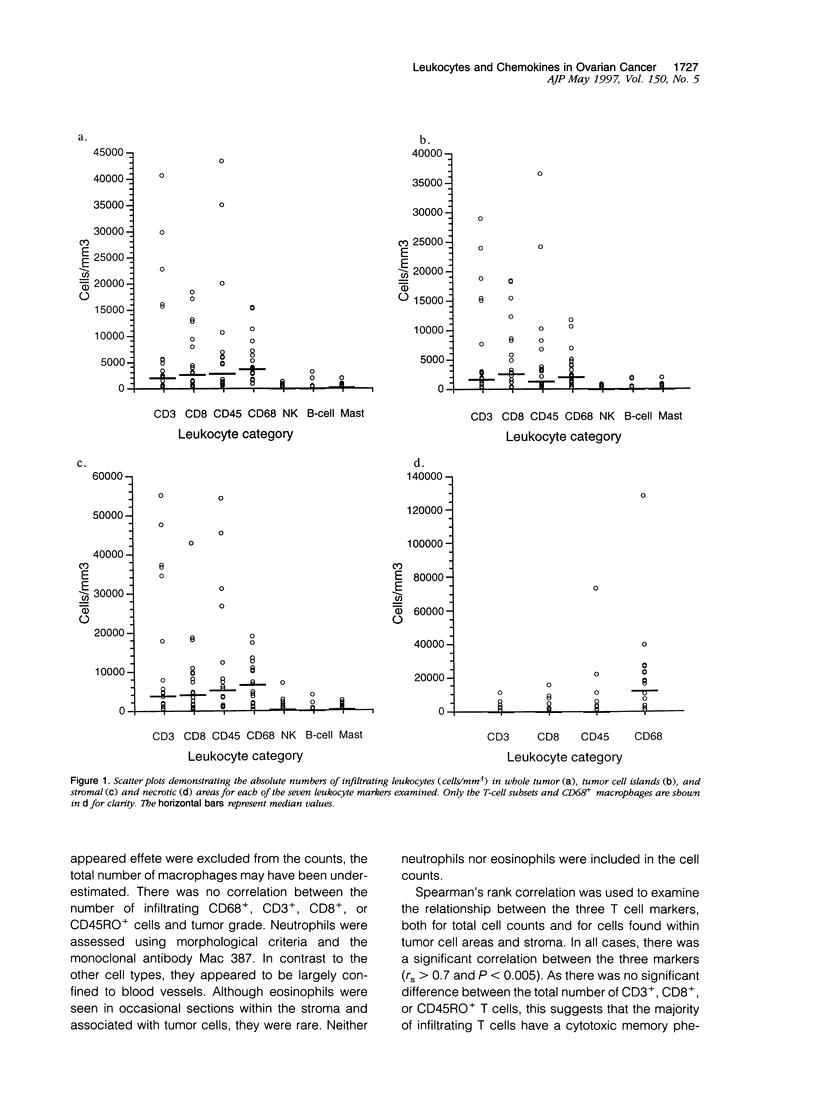
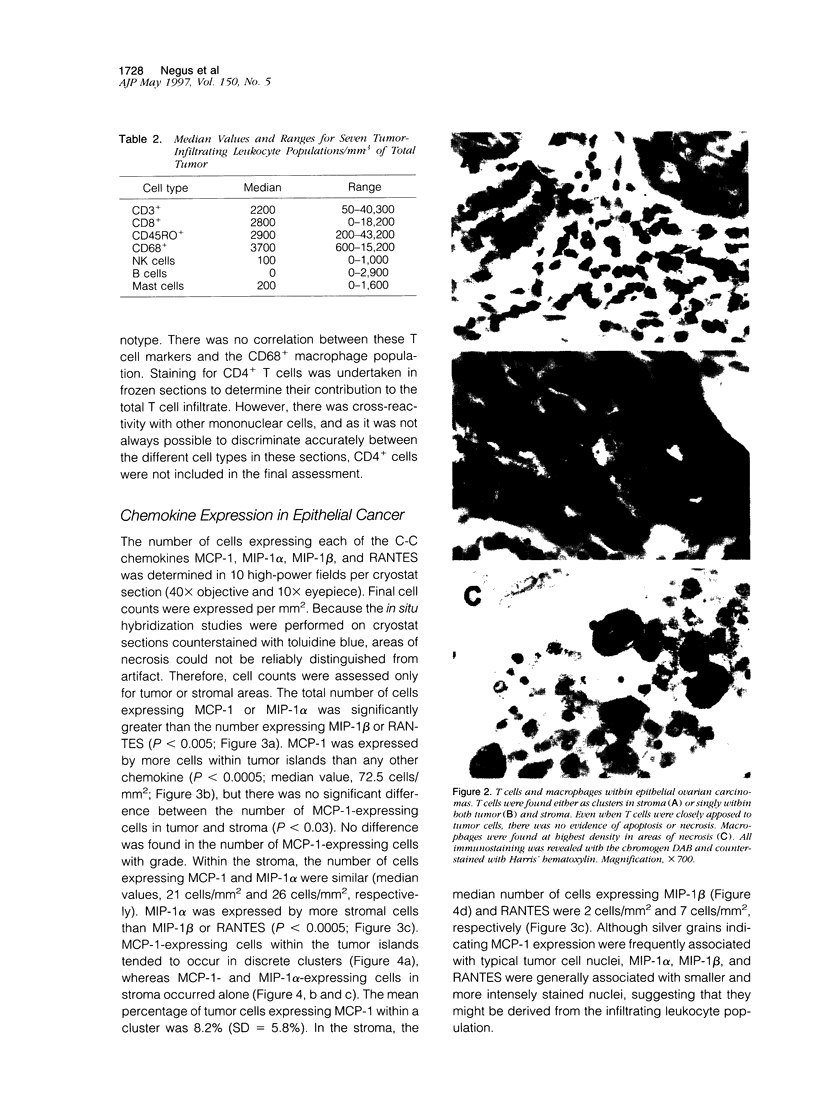
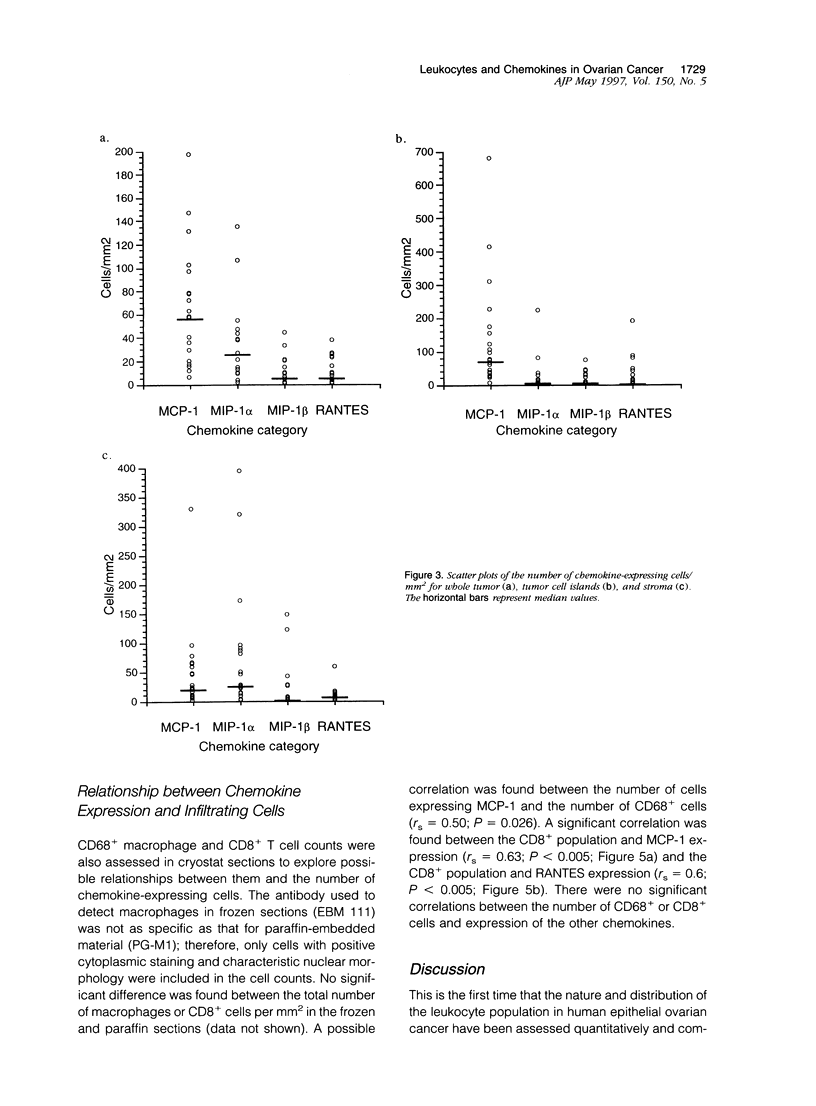
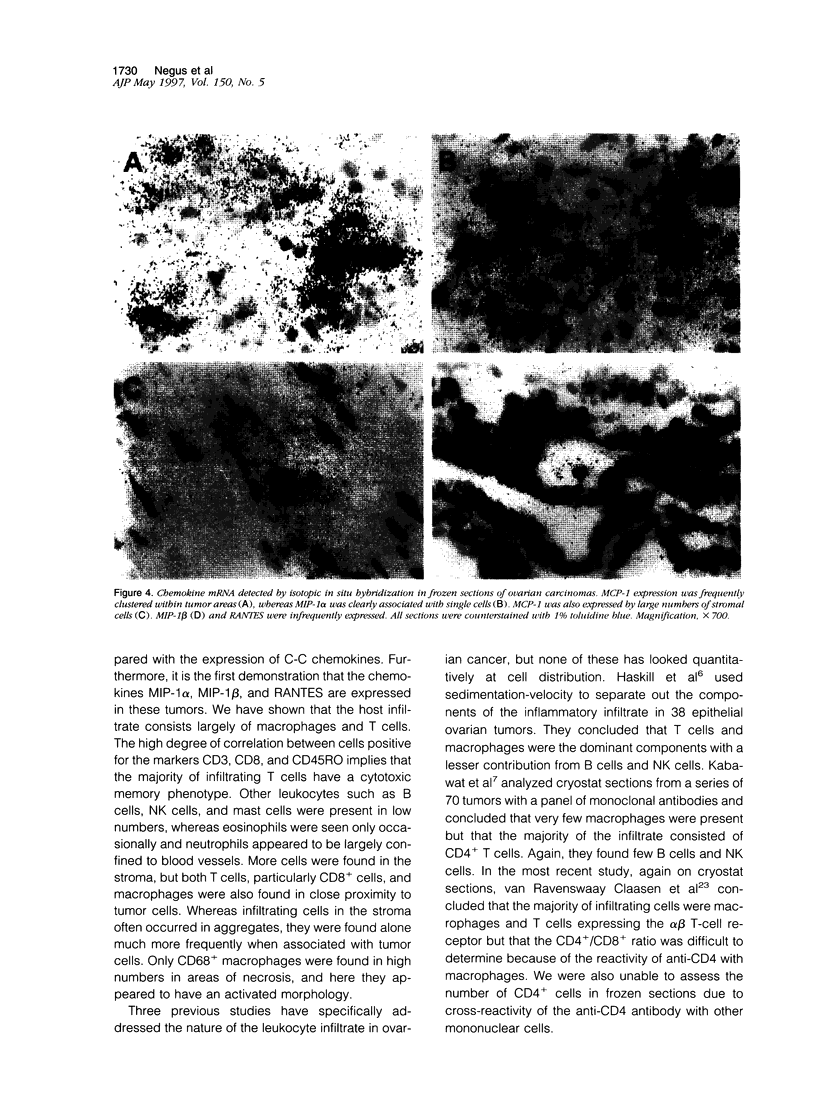
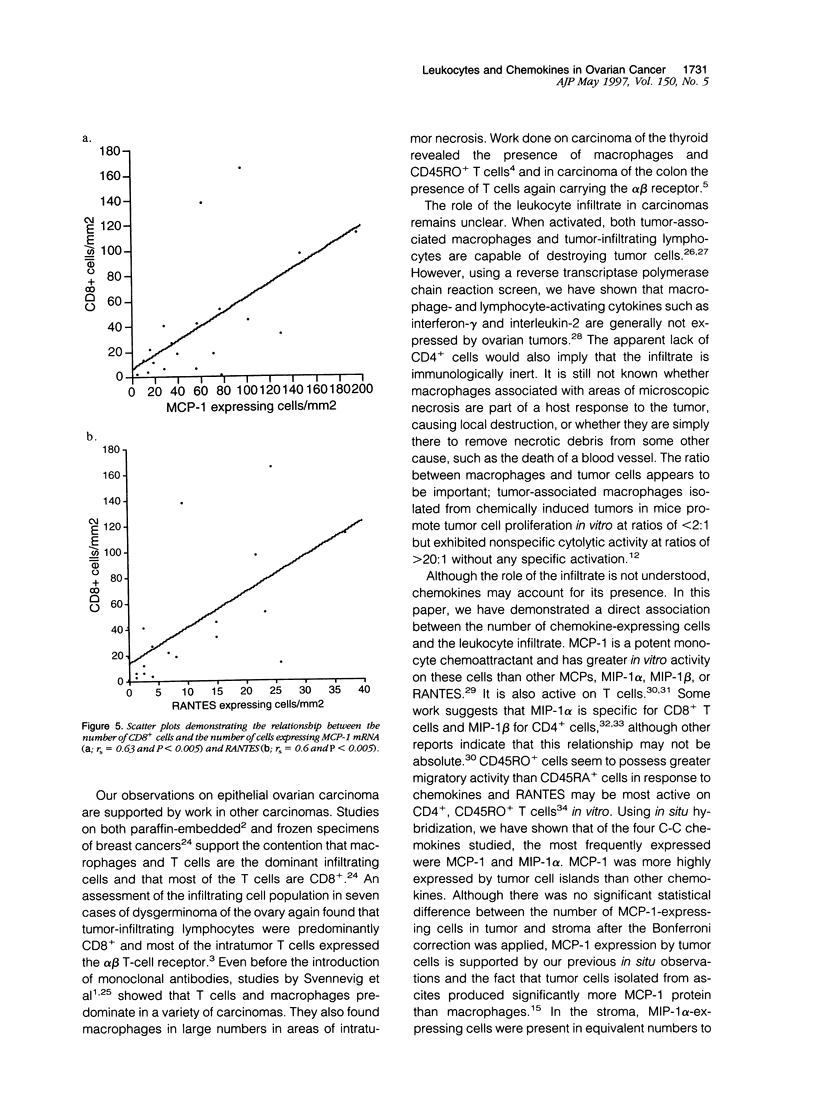
We have defined the host leukocyte infiltrate in epithelial ovarian tumors and related this to the expression of C-C chemokines. Immunohistochemical analysis of 20 paraffin-embedded biopsies showed that the infiltrate was primarily composed of CD68+ macrophages and CD8+/CD45RO+ T cells (median values, 3700 cells/mm3 and 2200 cells/mm3, respectively). Natural killer cells, B cells, and mast cells occurred in lower numbers (median values, 0 to 200 cells/mm3). Eosinophils were rarely seen and neutrophils were mainly confined to blood vessels. More infiltrating cells were found in stromal than in tumor areas. Only macrophages occurred in significant numbers in areas of necrosis (P < 0.0005). Using in situ hybridization to mRNA, we examined expression of the chemokines MCP-1, MIP-1 alpha, MIP-1 beta, and RANTES. MCP-1 and MIP-1 alpha were expressed by significantly more cells than MIP-1 beta and RANTES (P < 0.005). In tumor epithelial areas, the predominant chemokine was MCP-1. MCP-1 and MIP-1 alpha were the predominant stromal chemokines. A significant correlation was found between the total number of CD8+ T cells and the number of cells expressing MCP-1 (rs = 0.63 and P < 0.003, respectively) and between the CD8+ population and RANTES-expressing cells (rs = 0.6 and P < 0.003). A correlation was also found between CD68+ macrophages and the number of cells expressing MCP-1 (rs = 0.50 and P = 0.026). We suggest that MCP-1 may be responsible for the leukocyte infiltrate in ovarian carcinomas, but the expression of other chemokines may determine its exact nature.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apiranthitou-Drogari M., Paganin C., Bernasconi S., Losa G., Maneo A., Colombo N., Mantovani A., Allavena P. In search of specific cytotoxic T lymphocytes infiltrating or accompanying human ovarian carcinoma. Cancer Immunol Immunother. 1992;35(4):289–295. doi: 10.1007/BF01789337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini M., Kiderlen A. F., Decker T., Lohmann-Matthes M. L. Functional heterogeneity of murine macrophage precursor cells from spleen and bone marrow. Cell Immunol. 1986 Sep;101(2):339–350. doi: 10.1016/0008-8749(86)90147-4. [DOI] [PubMed] [Google Scholar]
- Banner B. F., Savas L., Baker S., Woda B. A. Characterization of the inflammatory cell populations in normal colon and colonic carcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64(4):213–220. doi: 10.1007/BF02915115. [DOI] [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. Multiple significance tests: the Bonferroni method. BMJ. 1995 Jan 21;310(6973):170–170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke F., Relf M., Negus R., Balkwill F. A cytokine profile of normal and malignant ovary. Cytokine. 1996 Jul;8(7):578–585. doi: 10.1006/cyto.1996.0077. [DOI] [PubMed] [Google Scholar]
- CURTIS A. S. Area and volume measurements by random sampling methods. Med Biol Illus. 1960 Oct;10:261–266. [PubMed] [Google Scholar]
- Dietl J., Horny H. P., Ruck P., Kaiserling E. Dysgerminoma of the ovary. An immunohistochemical study of tumor-infiltrating lymphoreticular cells and tumor cells. Cancer. 1993 Apr 15;71(8):2562–2568. doi: 10.1002/1097-0142(19930415)71:8<2562::aid-cncr2820710821>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Macrophages and metastasis--a biological approach to cancer therapy. Cancer Res. 1985 Oct;45(10):4714–4726. [PubMed] [Google Scholar]
- Fidler I. J., Schroit A. J. Synergism between lymphokines and muramyl dipeptide encapsulated in liposomes: in situ activation of macrophages and therapy of spontaneous cancer metastases. J Immunol. 1984 Jul;133(1):515–518. [PubMed] [Google Scholar]
- Foulkes W. D., Ragoussis J., Stamp G. W., Allan G. J., Trowsdale J. Frequent loss of heterozygosity on chromosome 6 in human ovarian carcinoma. Br J Cancer. 1993 Mar;67(3):551–559. doi: 10.1038/bjc.1993.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen T. M., Huang S., Curnutte J., Fowler P., Martinez V., Wehr C. M., Ames B. N., Chisari F. V. Extensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12808–12812. doi: 10.1073/pnas.91.26.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann G., Schumm-Draeger P. M., Müller C., Atai E., Wenzel B., Fabian T., Usadel K. H., Hübner K. T lymphocytes, CD68-positive cells and vascularisation in thyroid carcinomas. J Cancer Res Clin Oncol. 1994;120(11):651–656. doi: 10.1007/BF01245376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. C., Charles I. G., Thomsen L. L., Moss D. W., Holmes L. S., Baylis S. A., Rhodes P., Westmore K., Emson P. C., Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabawat S. E., Bast R. C., Jr, Welch W. R., Knapp R. C., Bhan A. K. Expression of major histocompatibility antigens and nature of inflammatory cellular infiltrate in ovarian neoplasms. Int J Cancer. 1983 Nov 15;32(5):547–554. doi: 10.1002/ijc.2910320505. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med. 1995 Feb;27(1):79–85. doi: 10.3109/07853899509031941. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M., Carter D., Mittal K., Yee L. D., Scata K. A., Donofrio L., Chambers S. K., Wang K. I., Yang-Feng T., Rohrschneider L. R. Ovarian adenocarcinomas express fms-complementary transcripts and fms antigen, often with coexpression of CSF-1. Am J Pathol. 1990 Jul;137(1):135–147. [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Loetscher P., Seitz M., Clark-Lewis I., Baggiolini M., Moser B. Monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J. 1994 Oct;8(13):1055–1060. doi: 10.1096/fasebj.8.13.7926371. [DOI] [PubMed] [Google Scholar]
- Lwin K. Y., Zuccarini O., Sloane J. P., Beverley P. C. An immunohistological study of leukocyte localization in benign and malignant breast tissue. Int J Cancer. 1985 Oct 15;36(4):433–438. doi: 10.1002/ijc.2910360404. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Bottazzi B., Colotta F., Sozzani S., Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992 Jul;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- Mantovani A. In vitro effects on tumor cells of macrophages isolated from an early-passage chemically-induced murine sarcoma and from its spontaneous metastases. Int J Cancer. 1981 Feb 15;27(2):221–228. doi: 10.1002/ijc.2910270215. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Tumor-associated macrophages in neoplastic progression: a paradigm for the in vivo function of chemokines. Lab Invest. 1994 Jul;71(1):5–16. [PubMed] [Google Scholar]
- Naylor M. S., Stamp G. W., Foulkes W. D., Eccles D., Balkwill F. R. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J Clin Invest. 1993 May;91(5):2194–2206. doi: 10.1172/JCI116446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus R. P., Stamp G. W., Relf M. G., Burke F., Malik S. T., Bernasconi S., Allavena P., Sozzani S., Mantovani A., Balkwill F. R. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995 May;95(5):2391–2396. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Sunday M. E. Suppression of tumor formation in vivo by expression of the JE gene in malignant cells. Mol Cell Biol. 1991 Jun;11(6):3125–3131. doi: 10.1128/mcb.11.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. J., Carr M. W., Springer T. A. C-C chemokines, but not the C-X-C chemokines interleukin-8 and interferon-gamma inducible protein-10, stimulate transendothelial chemotaxis of T lymphocytes. Eur J Immunol. 1995 Dec;25(12):3482–3488. doi: 10.1002/eji.1830251241. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Camp R. D., Kaspari J. W., Goeddel D. V. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993 Jun 1;177(6):1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Toy K. J., Goeddel D. V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990 Oct 18;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Scholl S. M., Pallud C., Beuvon F., Hacene K., Stanley E. R., Rohrschneider L., Tang R., Pouillart P., Lidereau R. Anti-colony-stimulating factor-1 antibody staining in primary breast adenocarcinomas correlates with marked inflammatory cell infiltrates and prognosis. J Natl Cancer Inst. 1994 Jan 19;86(2):120–126. doi: 10.1093/jnci/86.2.120. [DOI] [PubMed] [Google Scholar]
- Svennevig J. L., Lövik M., Svaar H. Isolation and characterization of lymphocytes and macrophages from solid, malignant human tumours. Int J Cancer. 1979 May 15;23(5):626–631. doi: 10.1002/ijc.2910230507. [DOI] [PubMed] [Google Scholar]
- Svennevig J. L., Svaar H. Content and distribution of macrophages and lymphocytes in solid malignant human tumours. Int J Cancer. 1979 Dec 15;24(6):754–758. doi: 10.1002/ijc.2910240609. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Kuratsu J., Takeya M., Yoshimura T., Ushio Y. Expression and localization of messenger RNA and protein for monocyte chemoattractant protein-1 in human malignant glioma. J Neurosurg. 1994 Jun;80(6):1056–1062. doi: 10.3171/jns.1994.80.6.1056. [DOI] [PubMed] [Google Scholar]
- Tang R., Beuvon F., Ojeda M., Mosseri V., Pouillart P., Scholl S. M-CSF (monocyte colony stimulating factor) and M-CSF receptor expression by breast tumour cells: M-CSF mediated recruitment of tumour infiltrating monocytes? J Cell Biochem. 1992 Dec;50(4):350–356. doi: 10.1002/jcb.240500403. [DOI] [PubMed] [Google Scholar]
- Taub D. D., Conlon K., Lloyd A. R., Oppenheim J. J., Kelvin D. J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993 Apr 16;260(5106):355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Uguccioni M., D'Apuzzo M., Loetscher M., Dewald B., Baggiolini M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1 alpha and MIP-1 beta on human monocytes. Eur J Immunol. 1995 Jan;25(1):64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Griffin J. D., Rambaldi A., Chen Z. G., Mantovani A. Induction of monocyte migration by recombinant macrophage colony-stimulating factor. J Immunol. 1988 Jul 15;141(2):575–579. [PubMed] [Google Scholar]
- van Ravenswaay Claasen H. H., Kluin P. M., Fleuren G. J. Tumor infiltrating cells in human cancer. On the possible role of CD16+ macrophages in antitumor cytotoxicity. Lab Invest. 1992 Aug;67(2):166–174. [PubMed] [Google Scholar]