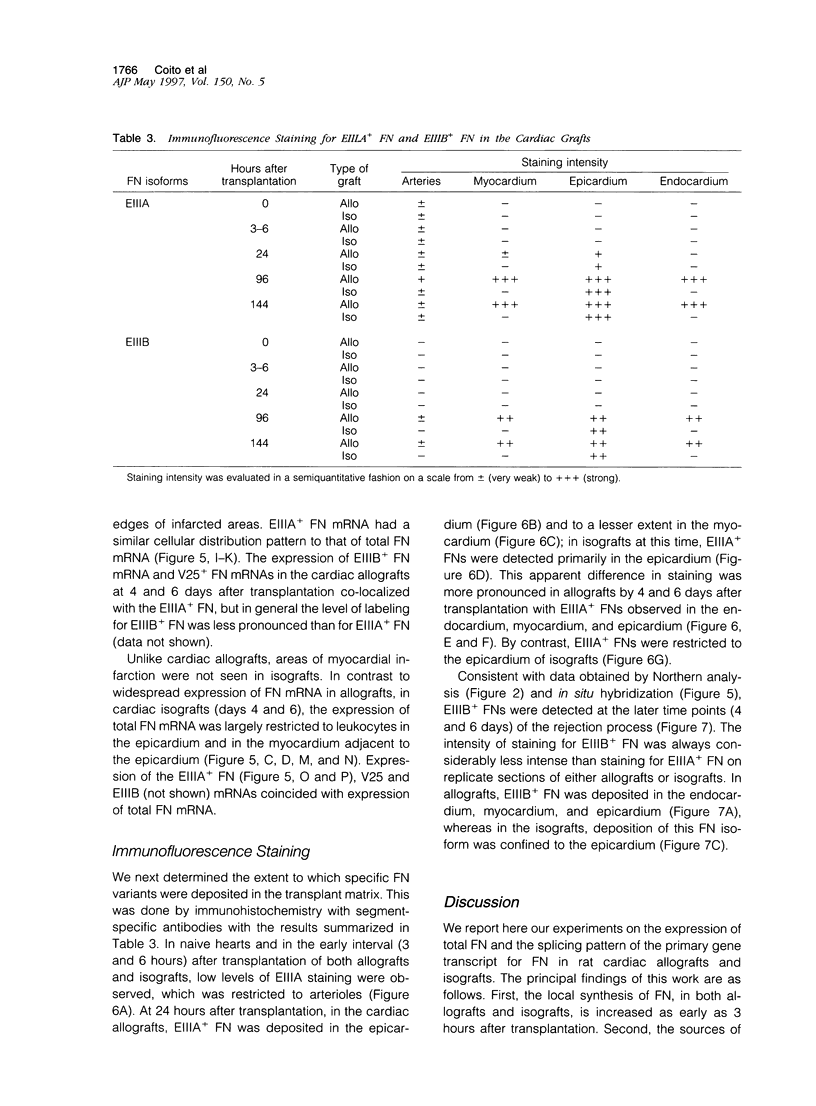
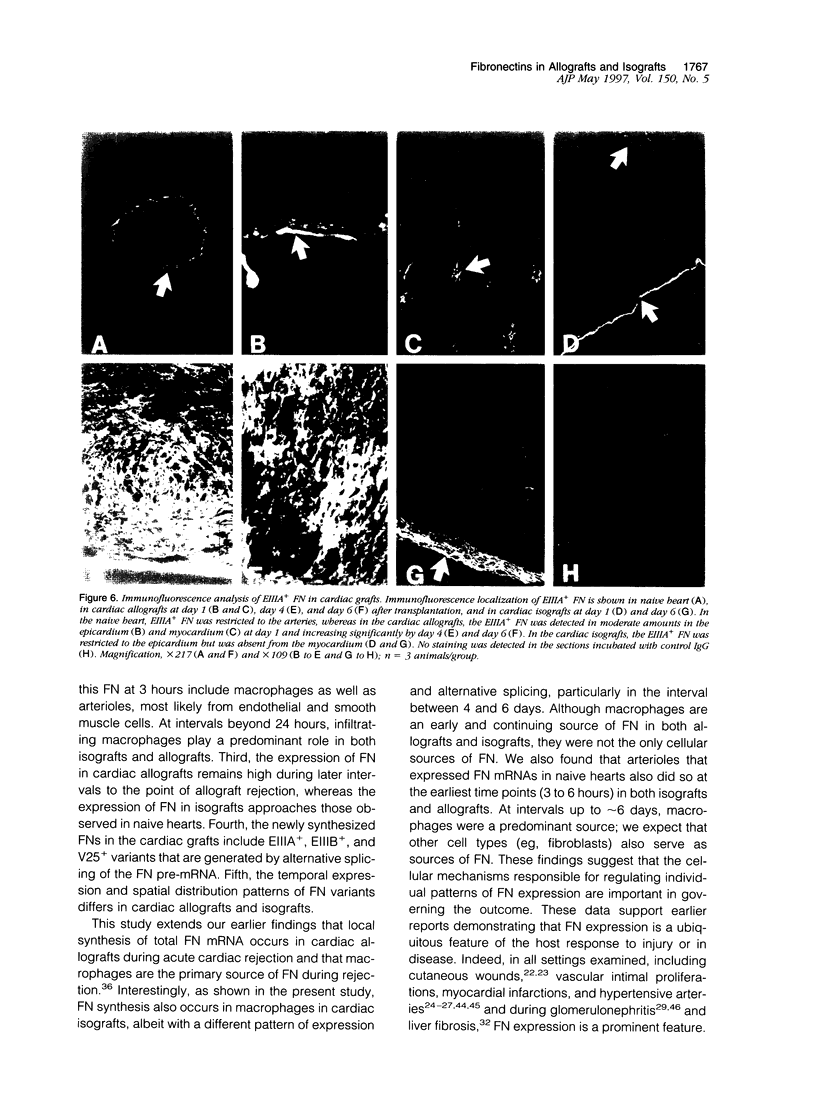
Abstract
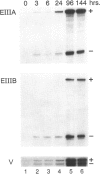
Allograft rejection is associated with infiltration of inflammatory cells and deposition of extracellular matrix proteins. The extent to which diversity in the extracellular matrix regulates inflammatory cell function in transplants remains unclear. One group of extracellular matrix proteins, termed fibronectins (FNs), exhibits inherent diversity as a consequence of alternative splicing in three segments: EIIIA, EIIIB, or V. Although the EIIIA segment has documented functions in mesenchymal cell differentiation, neither this segment nor the EIIIB segment have been tested for effects specific to leukocyte functions. By contrast, the V region can include the CS-1 segment to which leukocytes may adhere through alpha 4 beta 1 integrins. In this study, we demonstrate that EIIIA+, EIIIB+, and V+ FN variants are synthesized, primarily by macrophages in distinct temporal and spatial patterns in two rat cardiac transplant models: either with antigenic challenge, allografts, or without challenge, isografts. The ratio of EIIIA inclusion into FN increases by day 1 in allografts and isografts and remains high until allografts are rejected (approximately 7 days) but falls to normal levels in tolerated isografts (day 6). EIIIB+ FN ratios in allografts peak later than do EIIIA+ FNs (day 4). EIIIB+ FN ratios remain relatively low in isografts. Interestingly, EIIIA+ and EIIIB+ FNs are deposited prominently in the myocardium of rejecting allografts in close association with infiltrating leukocytes, and FN expression and deposition are prominent at sites of infarction. By contrast, these FNs are largely restricted to the epicardium and to a lesser degree in the immediately adjacent myocardium in isografts. CS-1+ FNs increase in allografts and isografts at 3 hours after transplantation but are particularly prominent in allografts 1 to 3 days before rejection. Our data suggest that FN splicing variants have a differential role in the effector functions of leukocytes in allografts and isografts and provide a foundation for testing their function on leukocytes and a rationale for FN-based therapeutics to modulate allograft rejection in transplant recipients.
Full text
PDF


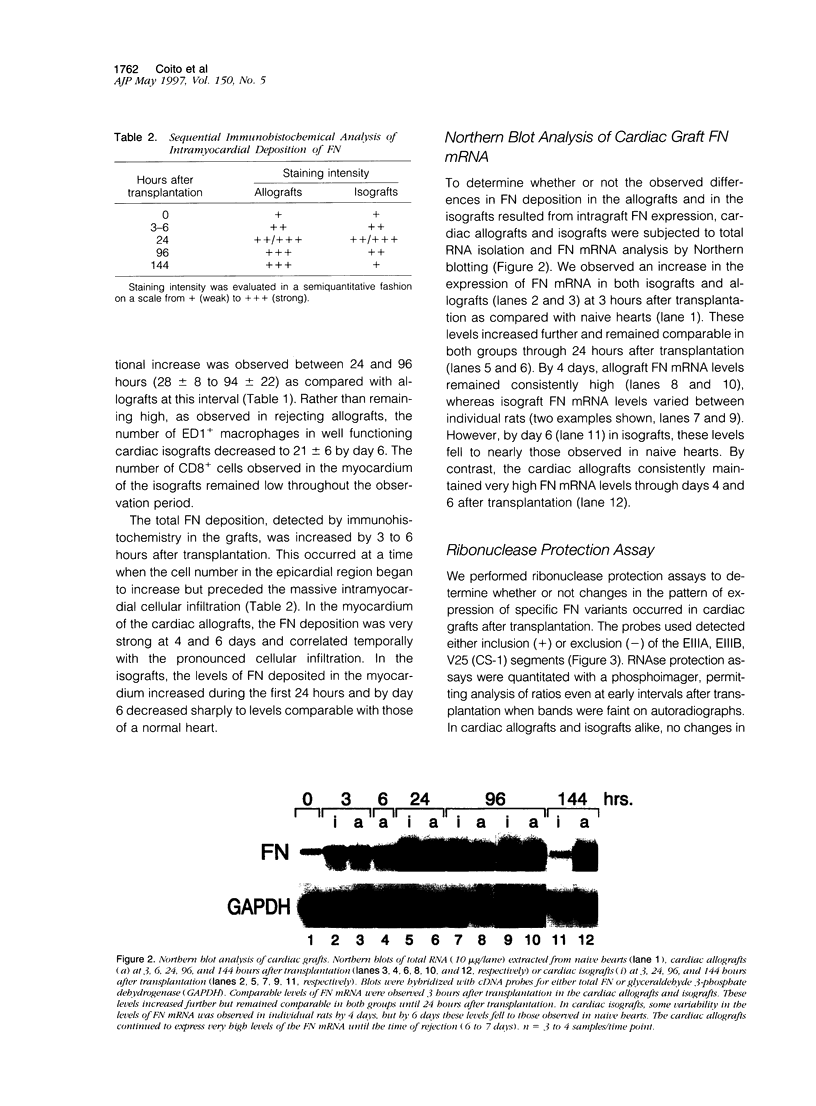
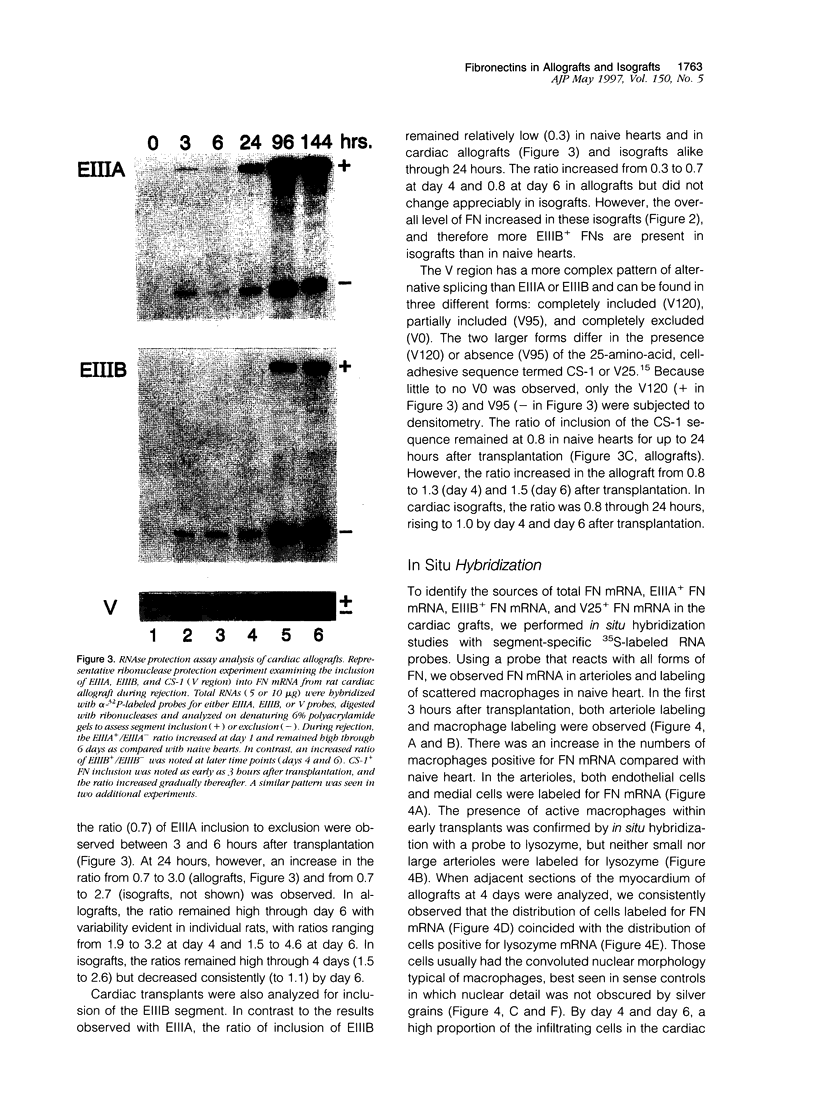
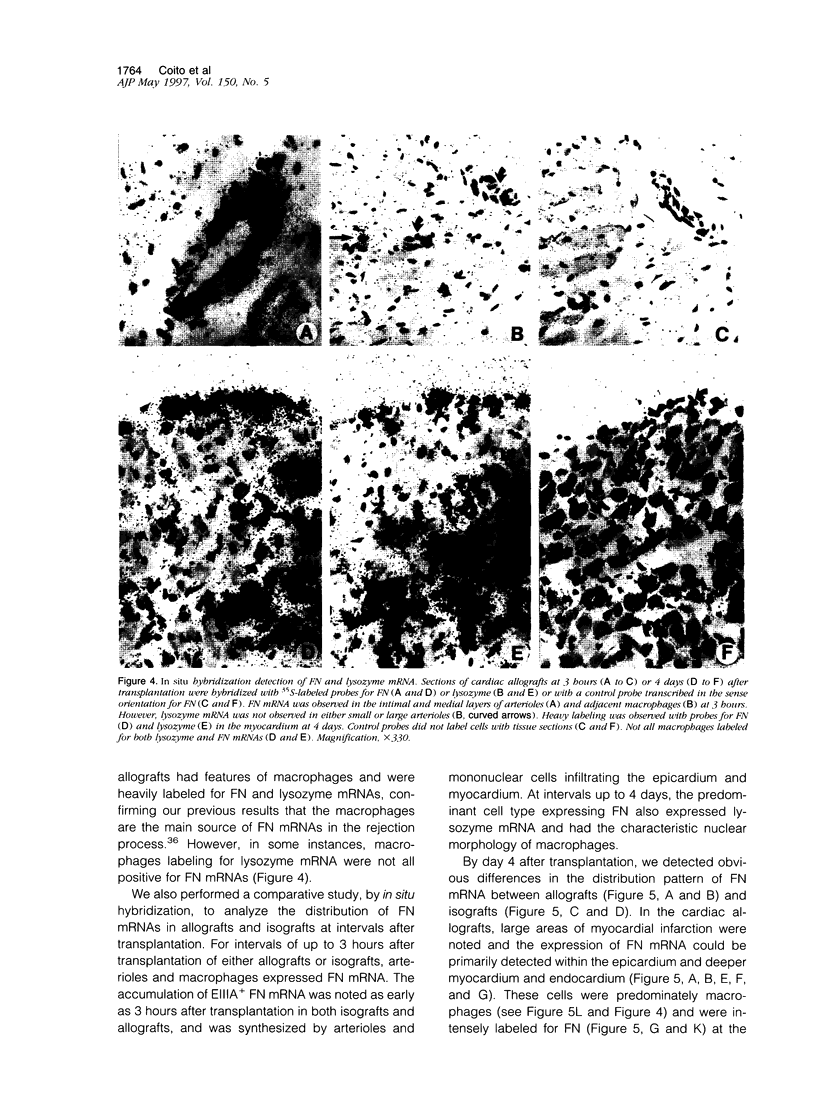
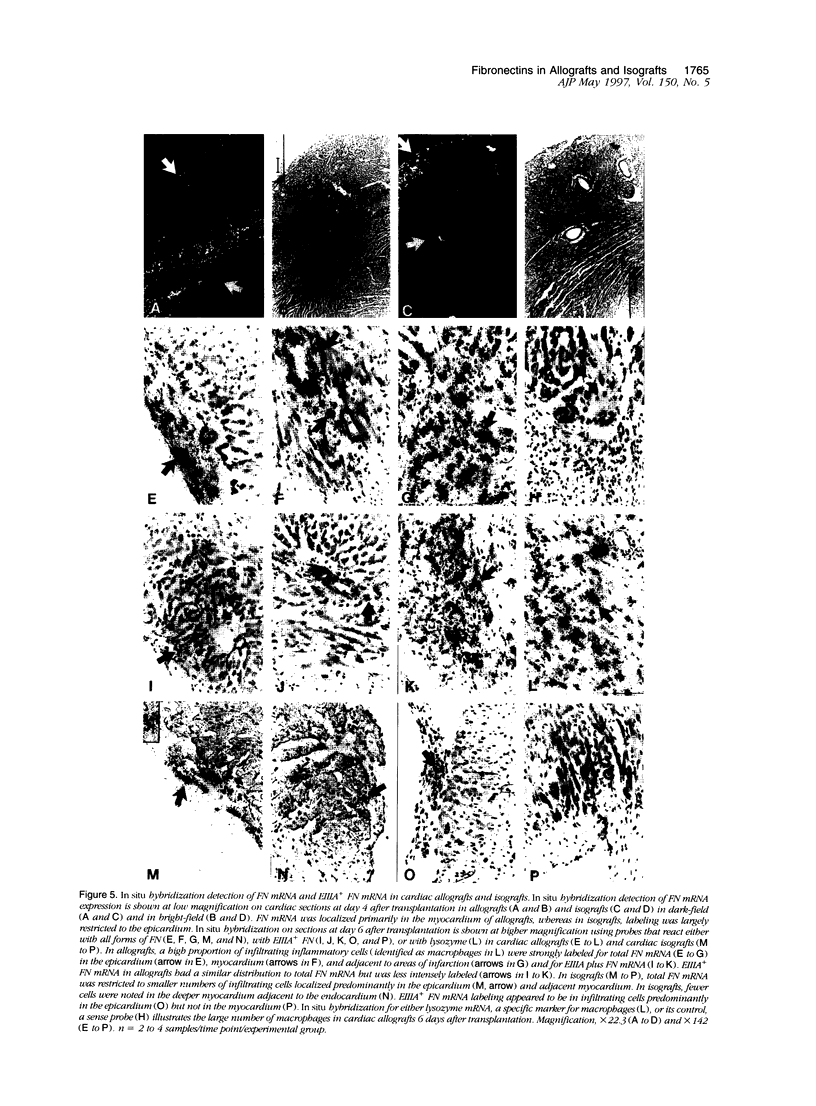





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes J. L., Hastings R. R., De la Garza M. A. Sequential expression of cellular fibronectin by platelets, macrophages, and mesangial cells in proliferative glomerulonephritis. Am J Pathol. 1994 Sep;145(3):585–597. [PMC free article] [PubMed] [Google Scholar]
- Barnes J. L., Torres E. S., Mitchell R. J., Peters J. H. Expression of alternatively spliced fibronectin variants during remodeling in proliferative glomerulonephritis. Am J Pathol. 1995 Nov;147(5):1361–1371. [PMC free article] [PubMed] [Google Scholar]
- Borsi L., Carnemolla B., Castellani P., Rosellini C., Vecchio D., Allemanni G., Chang S. E., Taylor-Papadimitriou J., Pande H., Zardi L. Monoclonal antibodies in the analysis of fibronectin isoforms generated by alternative splicing of mRNA precursors in normal and transformed human cells. J Cell Biol. 1987 Mar;104(3):595–600. doi: 10.1083/jcb.104.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. F., Dubin D., Lavigne L., Logan B., Dvorak H. F., Van de Water L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol. 1993 Mar;142(3):793–801. [PMC free article] [PubMed] [Google Scholar]
- Brown L. F., Olbricht S. M., Berse B., Jackman R. W., Matsueda G., Tognazzi K. A., Manseau E. J., Dvorak H. F., Van de Water L. Overexpression of vascular permeability factor (VPF/VEGF) and its endothelial cell receptors in delayed hypersensitivity skin reactions. J Immunol. 1995 Mar 15;154(6):2801–2807. [PubMed] [Google Scholar]
- Brown L. F., Yeo K. T., Berse B., Yeo T. K., Senger D. R., Dvorak H. F., van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992 Nov 1;176(5):1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnemolla B., Balza E., Siri A., Zardi L., Nicotra M. R., Bigotti A., Natali P. G. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989 Mar;108(3):1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Dvorak H. F., Colvin R. B. Fibronectin in delayed-type hypersensitivity skin reactions: associations with vessel permeability and endothelial cell activation. J Immunol. 1981 Feb;126(2):787–793. [PubMed] [Google Scholar]
- Clausell N., Molossi S., Rabinovitch M. Increased interleukin-1 beta and fibronectin expression are early features of the development of the postcardiac transplant coronary arteriopathy in piglets. Am J Pathol. 1993 Jun;142(6):1772–1786. [PMC free article] [PubMed] [Google Scholar]
- Clausell N., Molossi S., Sett S., Rabinovitch M. In vivo blockade of tumor necrosis factor-alpha in cholesterol-fed rabbits after cardiac transplant inhibits acute coronary artery neointimal formation. Circulation. 1994 Jun;89(6):2768–2779. doi: 10.1161/01.cir.89.6.2768. [DOI] [PubMed] [Google Scholar]
- Coito A. J., Binder J., Brown L. F., de Sousa M., Van de Water L., Kupiec-Weglinski J. W. Anti-TNF-alpha treatment down-regulates the expression of fibronectin and decreases cellular infiltration of cardiac allografts in rats. J Immunol. 1995 Mar 15;154(6):2949–2958. [PubMed] [Google Scholar]
- Coito A. J., Binder J., de Sousa M., Kupiec-Weglinski J. W. The expression of extracellular matrix proteins during accelerated rejection of cardiac allografts in sensitized rats. Transplantation. 1994 Feb 27;57(4):599–605. [PubMed] [Google Scholar]
- Coito A. J., De Sousa M., Kupiec-Weglinski J. W. The role of cellular and extracellular matrix adhesion proteins in organ transplantation. Cell Adhes Commun. 1994 Jul;2(3):249–255. doi: 10.3109/15419069409004444. [DOI] [PubMed] [Google Scholar]
- Dubin D., Peters J. H., Brown L. F., Logan B., Kent K. C., Berse B., Berven S., Cercek B., Sharifi B. G., Pratt R. E. Balloon catheterization induced arterial expression of embryonic fibronectins. Arterioscler Thromb Vasc Biol. 1995 Nov;15(11):1958–1967. doi: 10.1161/01.atv.15.11.1958. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989 Jun;106(2):375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- George E. L., Georges-Labouesse E. N., Patel-King R. S., Rayburn H., Hynes R. O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993 Dec;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Gismondi A., Milella M., Palmieri G., Piccoli M., Frati L., Santoni A. Stimulation of protein tyrosine phosphorylation by interaction of NK cells with fibronectin via alpha 4 beta 1 and alpha 5 beta 1. J Immunol. 1995 Apr 1;154(7):3128–3137. [PubMed] [Google Scholar]
- Glukhova M. A., Frid M. G., Shekhonin B. V., Vasilevskaya T. D., Grunwald J., Saginati M., Koteliansky V. E. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol. 1989 Jul;109(1):357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F., Billingham R. E., Burgess L. Distribution of fibronectin during wound healing in vivo. J Invest Dermatol. 1981 Mar;76(3):181–189. doi: 10.1111/1523-1747.ep12525694. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Hershkoviz R., Cahalon L., Gilat D., Miron S., Miller A., Lider O. Physically damaged extracellular matrix induces TNF-alpha secretion by interacting resting CD4+ T cells and macrophages. Scand J Immunol. 1993 Jan;37(1):111–115. doi: 10.1111/j.1365-3083.1993.tb01672.x. [DOI] [PubMed] [Google Scholar]
- Hershkoviz R., Gilat D., Miron S., Mekori Y. A., Aderka D., Wallach D., Vlodavsky I., Cohen I. R., Lider O. Extracellular matrix induces tumour necrosis factor-alpha secretion by an interaction between resting rat CD4+ T cells and macrophages. Immunology. 1993 Jan;78(1):50–57. [PMC free article] [PubMed] [Google Scholar]
- Hines K. L., Kulkarni A. B., McCarthy J. B., Tian H., Ward J. M., Christ M., McCartney-Francis N. L., Furcht L. T., Karlsson S., Wahl S. M. Synthetic fibronectin peptides interrupt inflammatory cell infiltration in transforming growth factor beta 1 knockout mice. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5187–5191. doi: 10.1073/pnas.91.11.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Jarnagin W. R., Rockey D. C., Koteliansky V. E., Wang S. S., Bissell D. M. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994 Dec;127(6 Pt 2):2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton A. A., Connelly C. M., Romo G. M., Mamuya W., Apstein C. S., Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest. 1992 Apr;89(4):1060–1068. doi: 10.1172/JCI115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., De Sousa M. Lymphocyte traffic is modified in vivo by anti-laminin antibody. Immunology. 1991 Feb;72(2):312–313. [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Hemler M. E. The pathophysiologic role of alpha 4 integrins in vivo. J Clin Invest. 1994 Nov;94(5):1722–1728. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamuya W. S., Brecher P. Fibronectin expression in the normal and hypertrophic rat heart. J Clin Invest. 1992 Feb;89(2):392–401. doi: 10.1172/JCI115598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Yamada A., Kay J., Yamada K. M., Akiyama S. K., Schlossman S. F., Morimoto C. Activation of CD4 cells by fibronectin and anti-CD3 antibody. A synergistic effect mediated by the VLA-5 fibronectin receptor complex. J Exp Med. 1989 Oct 1;170(4):1133–1148. doi: 10.1084/jem.170.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M. F., O'Rourke A. M., Champoux P., Kane K. P. Equilibrium binding of cytotoxic T lymphocytes to class I antigen. J Immunol. 1991 Jul 1;147(1):36–41. [PubMed] [Google Scholar]
- Molossi S., Elices M., Arrhenius T., Diaz R., Coulber C., Rabinovitch M. Blockade of very late antigen-4 integrin binding to fibronectin with connecting segment-1 peptide reduces accelerated coronary arteriopathy in rabbit cardiac allografts. J Clin Invest. 1995 Jun;95(6):2601–2610. doi: 10.1172/JCI117962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molossi S., Elices M., Arrhenius T., Rabinovitch M. Lymphocyte transendothelial migration toward smooth muscle cells in interleukin-1 beta-stimulated co-cultures is related to fibronectin interactions with alpha 4 beta 1 and alpha 5 beta 1 integrins. J Cell Physiol. 1995 Sep;164(3):620–633. doi: 10.1002/jcp.1041640321. [DOI] [PubMed] [Google Scholar]
- Mould A. P., Wheldon L. A., Komoriya A., Wayner E. A., Yamada K. M., Humphries M. J. Affinity chromatographic isolation of the melanoma adhesion receptor for the IIICS region of fibronectin and its identification as the integrin alpha 4 beta 1. J Biol Chem. 1990 Mar 5;265(7):4020–4024. [PubMed] [Google Scholar]
- Nickeleit V., Zagachin L., Nishikawa K., Peters J. H., Hynes R. O., Colvin R. B. Embryonic fibronectin isoforms are synthesized in crescents in experimental autoimmune glomerulonephritis. Am J Pathol. 1995 Oct;147(4):965–978. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992 Jan 10;68(1):1–2. doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- Ostergaard H. L., Ma E. A. Fibronectin induces phosphorylation of a 120-kDa protein and synergizes with the T cell receptor to activate cytotoxic T cell clones. Eur J Immunol. 1995 Jan;25(1):252–256. doi: 10.1002/eji.1830250141. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Chen G. E., Hynes R. O. Fibronectin isoform distribution in the mouse. II. Differential distribution of the alternatively spliced EIIIB, EIIIA, and V segments in the adult mouse. Cell Adhes Commun. 1996 Aug;4(2):127–148. doi: 10.3109/15419069609010767. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Samuel J. L., Barrieux A., Dufour S., Dubus I., Contard F., Koteliansky V., Farhadian F., Marotte F., Thiéry J. P., Rappaport L. Accumulation of fetal fibronectin mRNAs during the development of rat cardiac hypertrophy induced by pressure overload. J Clin Invest. 1991 Nov;88(5):1737–1746. doi: 10.1172/JCI115492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E. Alternative splicing of fibronectin: three variants, three functions. Bioessays. 1991 Oct;13(10):527–533. doi: 10.1002/bies.950131006. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Patel R. S., Fonda D., Hynes R. O. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987 Sep;6(9):2573–2580. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S. Lymphocyte interactions with extracellular matrix. FASEB J. 1991 Jun;5(9):2292–2299. doi: 10.1096/fasebj.5.9.1860621. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990 Apr;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Takasaki I., Chobanian A. V., Mamuya W. S., Brecher P. Hypertension induces alternatively spliced forms of fibronectin in rat aorta. Hypertension. 1992 Jul;20(1):20–25. doi: 10.1161/01.hyp.20.1.20. [DOI] [PubMed] [Google Scholar]
- Vartio T., Laitinen L., Närvänen O., Cutolo M., Thornell L. E., Zardi L., Virtanen I. Differential expression of the ED sequence-containing form of cellular fibronectin in embryonic and adult human tissues. J Cell Sci. 1987 Nov;88(Pt 4):419–430. doi: 10.1242/jcs.88.4.419. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., Hines K. L., Imamichi T., Wahl A. M., Furcht L. T., McCarthy J. B. Synthetic fibronectin peptides suppress arthritis in rats by interrupting leukocyte adhesion and recruitment. J Clin Invest. 1994 Aug;94(2):655–662. doi: 10.1172/JCI117382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P., Culp L. A. Adhesion activity in fibronectin's alternatively spliced domain EDa (EIIIA) and its neighboring type III repeats: oncogene-dependent regulation. Exp Cell Res. 1994 Jul;213(1):253–265. doi: 10.1006/excr.1994.1197. [DOI] [PubMed] [Google Scholar]
- Xia P., Culp L. A. Adhesion activity in fibronectin's alternatively spliced domain EDa (EIIIA): complementarity to plasma fibronectin functions. Exp Cell Res. 1995 Apr;217(2):517–527. doi: 10.1006/excr.1995.1117. [DOI] [PubMed] [Google Scholar]
- Yamada A., Nikaido T., Nojima Y., Schlossman S. F., Morimoto C. Activation of human CD4 T lymphocytes. Interaction of fibronectin with VLA-5 receptor on CD4 cells induces the AP-1 transcription factor. J Immunol. 1991 Jan 1;146(1):53–56. [PubMed] [Google Scholar]
- de Sousa M., Tilney N. L., Kupiec-Weglinski J. W. Recognition of self within self: specific lymphocyte positioning and the extracellular matrix. Immunol Today. 1991 Aug;12(8):262–266. doi: 10.1016/0167-5699(91)90123-B. [DOI] [PubMed] [Google Scholar]