Abstract
The usefulness of standard in situ hybridization for viral nucleic acid detection is occasionally limited by its sensitivity limit of 10 to 50 copies per cell. A modified version of the recently described signal amplification method, catalyzed reporter deposition (CARD), and its application to formalin-fixed cells and tissue sections is presented. Deposition of the reporter is facilitated by using horseradish peroxidase catalyzing the deposition of biotinylated tyramide on the location of the probe target. The biotin accumulation created is usually detected with streptavidin-labeled enzymes or fluorochromes. In the present investigation, this step was replaced by streptavidin-Nanogold and combined with silver acetate autometallography. This resulted in deep-black precipitation at positive in situ hybridized reaction sites. The sensitivity of this new approach was tested with a biotinylated, genomic probe specific for human papillomavirus (HPV)-16/18. SiHa cells, a cervical carcinoma-derived cell line with one to two HPV16 copies per cell, and 10 histologically confirmed cervical carcinomas were used for the study. All samples were previously HPV16 positive with solution polymerase chain reaction, but only two of the cervical carcinomas were positive with standard in situ hybridization with barely visible signals. When employing CARD-Nanogold, SiHa cells and 9 of 10 biopsies proved positive with marked signals. It is concluded that this nonisotopic method can detect single viral copies in situ in routinely fixed material and may have the potential to replace in situ polymerase chain reaction in many applications.
Full text
PDF
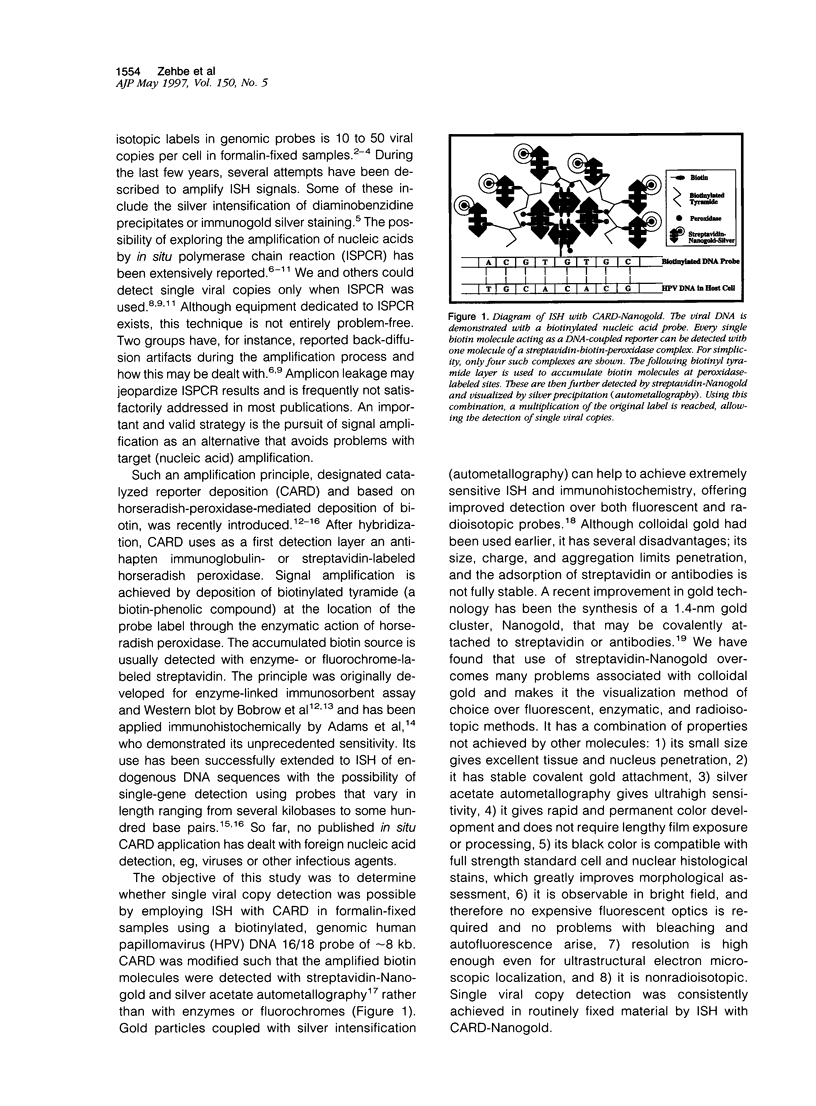


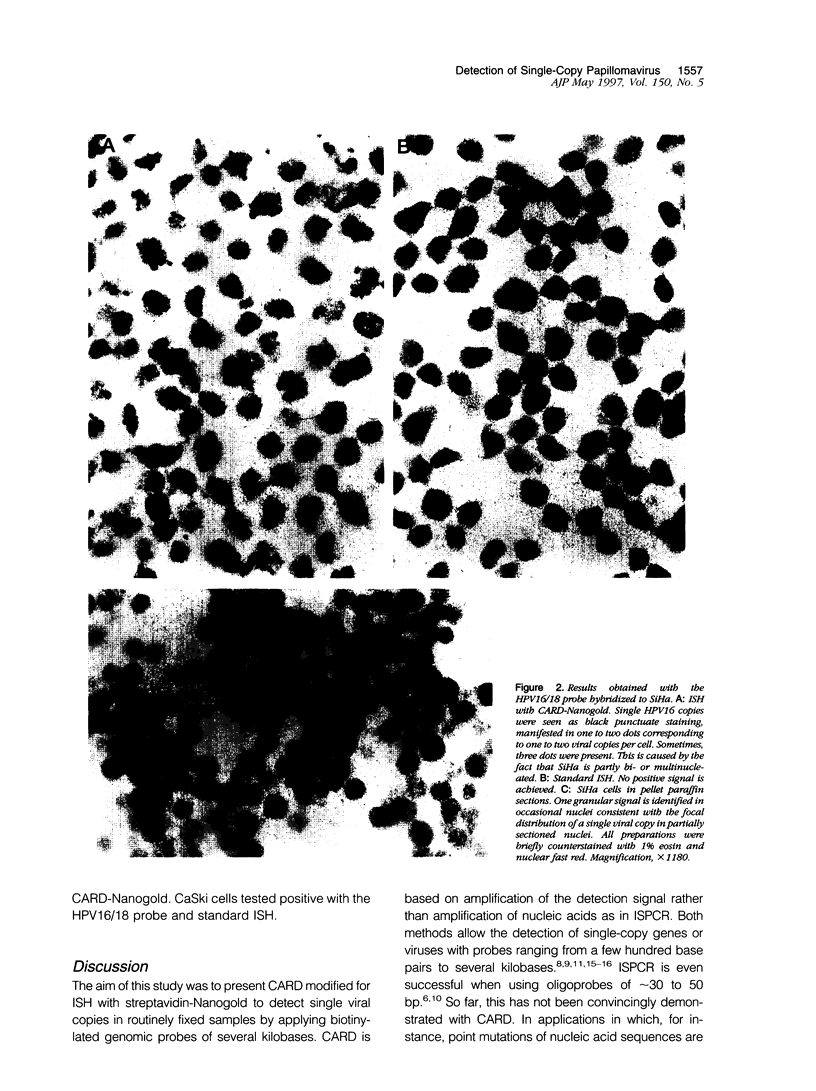
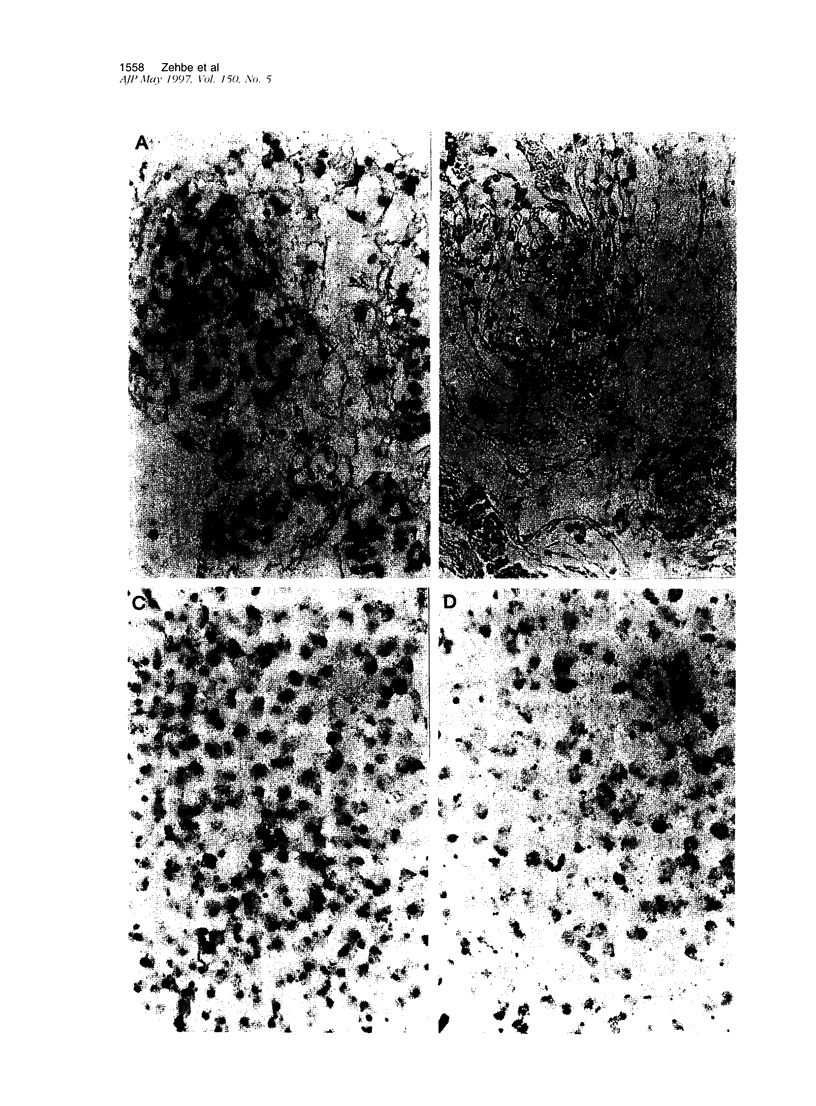



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem. 1992 Oct;40(10):1457–1463. doi: 10.1177/40.10.1527370. [DOI] [PubMed] [Google Scholar]
- Bagasra O., Hauptman S. P., Lischner H. W., Sachs M., Pomerantz R. J. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N Engl J Med. 1992 May 21;326(21):1385–1391. doi: 10.1056/NEJM199205213262103. [DOI] [PubMed] [Google Scholar]
- Bobrow M. N., Harris T. D., Shaughnessy K. J., Litt G. J. Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J Immunol Methods. 1989 Dec 20;125(1-2):279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- Bobrow M. N., Shaughnessy K. J., Litt G. J. Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. J Immunol Methods. 1991 Mar 1;137(1):103–112. doi: 10.1016/0022-1759(91)90399-z. [DOI] [PubMed] [Google Scholar]
- Burns J., Graham A. K., Frank C., Fleming K. A., Evans M. F., McGee J. O. Detection of low copy human papilloma virus DNA and mRNA in routine paraffin sections of cervix by non-isotopic in situ hybridisation. J Clin Pathol. 1987 Aug;40(8):858–864. doi: 10.1136/jcp.40.8.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl F., Kimura I., Osato T., Ito Y. Studies on a new human cell line (SiHa) derived from carcinoma of uterus. I. Its establishment and morphology. Proc Soc Exp Biol Med. 1970 Nov;135(2):543–545. doi: 10.3181/00379727-135-35091a. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Pardue M. L. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc Natl Acad Sci U S A. 1969 Jun;63(2):378–383. doi: 10.1073/pnas.63.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Retzel E. F., Staskus K. A. Amplification and detection of lentiviral DNA inside cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4971–4975. doi: 10.1073/pnas.87.13.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker G. W., Graf A. H., Hauser-Kronberger C., Wirnsberger G., Schiechl A., Bernatzky G., Wittauer U., Su H. C., Adam H., Thurner J. Application of silver acetate autometallography and gold-silver staining methods for in situ DNA hybridization. Chin Med J (Engl) 1993 Feb;106(2):83–92. [PubMed] [Google Scholar]
- Hainfeld J. F., Furuya F. R. A 1.4-nm gold cluster covalently attached to antibodies improves immunolabeling. J Histochem Cytochem. 1992 Feb;40(2):177–184. doi: 10.1177/40.2.1552162. [DOI] [PubMed] [Google Scholar]
- Kerstens H. M., Poddighe P. J., Hanselaar A. G. A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J Histochem Cytochem. 1995 Apr;43(4):347–352. doi: 10.1177/43.4.7897179. [DOI] [PubMed] [Google Scholar]
- Long A. A., Komminoth P., Lee E., Wolfe H. J. Comparison of indirect and direct in-situ polymerase chain reaction in cell preparations and tissue sections. Detection of viral DNA, gene rearrangements and chromosomal translocations. Histochemistry. 1993 Feb;99(2):151–162. doi: 10.1007/BF00571876. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., MacConnell P., Forde A., Delvenne P. Detection of human papillomavirus DNA in formalin-fixed tissues by in situ hybridization after amplification by polymerase chain reaction. Am J Pathol. 1991 Oct;139(4):847–854. [PMC free article] [PubMed] [Google Scholar]
- Patterson B. K., Till M., Otto P., Goolsby C., Furtado M. R., McBride L. J., Wolinsky S. M. Detection of HIV-1 DNA and messenger RNA in individual cells by PCR-driven in situ hybridization and flow cytometry. Science. 1993 May 14;260(5110):976–979. doi: 10.1126/science.8493534. [DOI] [PubMed] [Google Scholar]
- Pattillo R. A., Hussa R. O., Story M. T., Ruckert A. C., Shalaby M. R., Mattingly R. F. Tumor antigen and human chorionic gonadotropin in CaSki cells: a new epidermoid cervical cancer cell line. Science. 1977 Jun 24;196(4297):1456–1458. doi: 10.1126/science.867042. [DOI] [PubMed] [Google Scholar]
- Raap A. K., van de Corput M. P., Vervenne R. A., van Gijlswijk R. P., Tanke H. J., Wiegant J. Ultra-sensitive FISH using peroxidase-mediated deposition of biotin- or fluorochrome tyramides. Hum Mol Genet. 1995 Apr;4(4):529–534. doi: 10.1093/hmg/4.4.529. [DOI] [PubMed] [Google Scholar]
- Syrjänen S., Partanen P., Mäntyjärvi R., Syrjänen K. Sensitivity of in situ hybridization techniques using biotin- and 35S-labeled human papillomavirus (HPV) DNA probes. J Virol Methods. 1988 Mar-Apr;19(3-4):225–238. doi: 10.1016/0166-0934(88)90017-1. [DOI] [PubMed] [Google Scholar]
- Tourtellotte W. W., Verity A. N., Schmid P., Martinez S., Shapshak P. Covalent binding of formalin fixed paraffin embedded brain tissue sections to glass slides suitable for in situ hybridization. J Virol Methods. 1987 Feb;15(2):87–99. doi: 10.1016/0166-0934(87)90052-8. [DOI] [PubMed] [Google Scholar]
- Zehbe I., Rylander E., Strand A., Wilander E. Use of Probemix and OmniProbe biotinylated cDNA probes for detecting HPV infection in biopsy specimens from the genital tract. J Clin Pathol. 1993 May;46(5):437–440. doi: 10.1136/jcp.46.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]




