Abstract
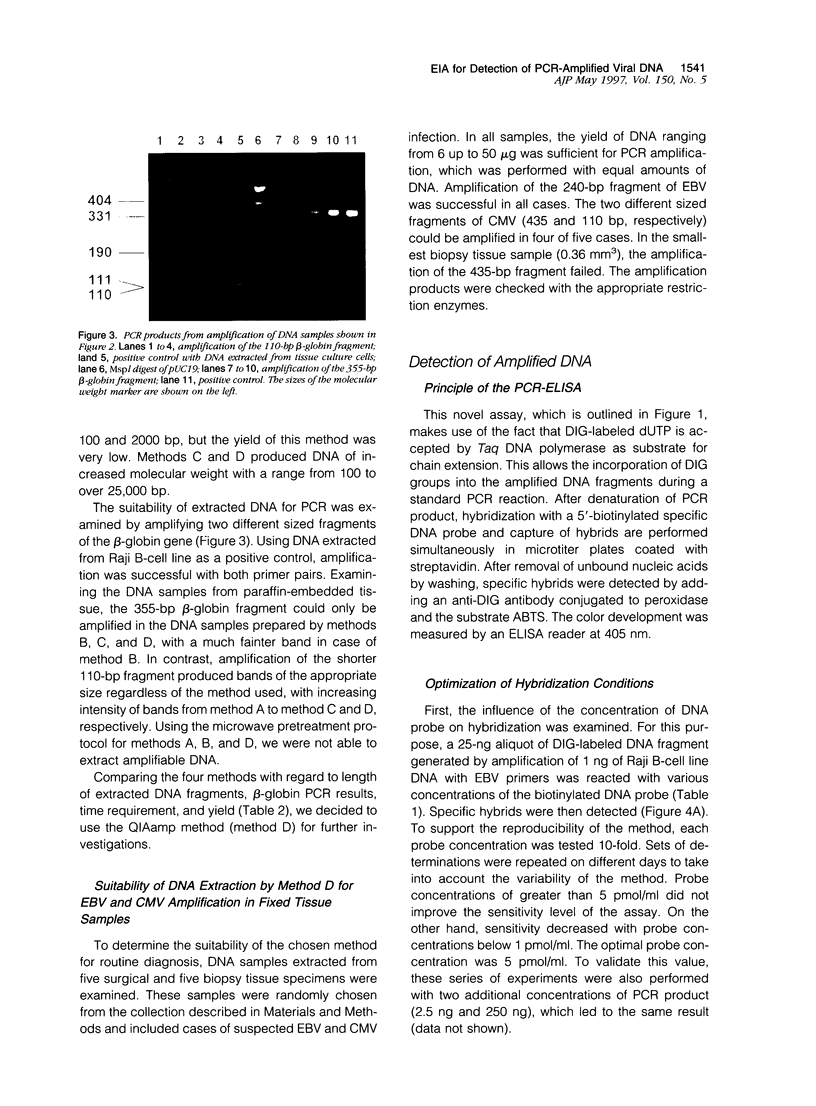
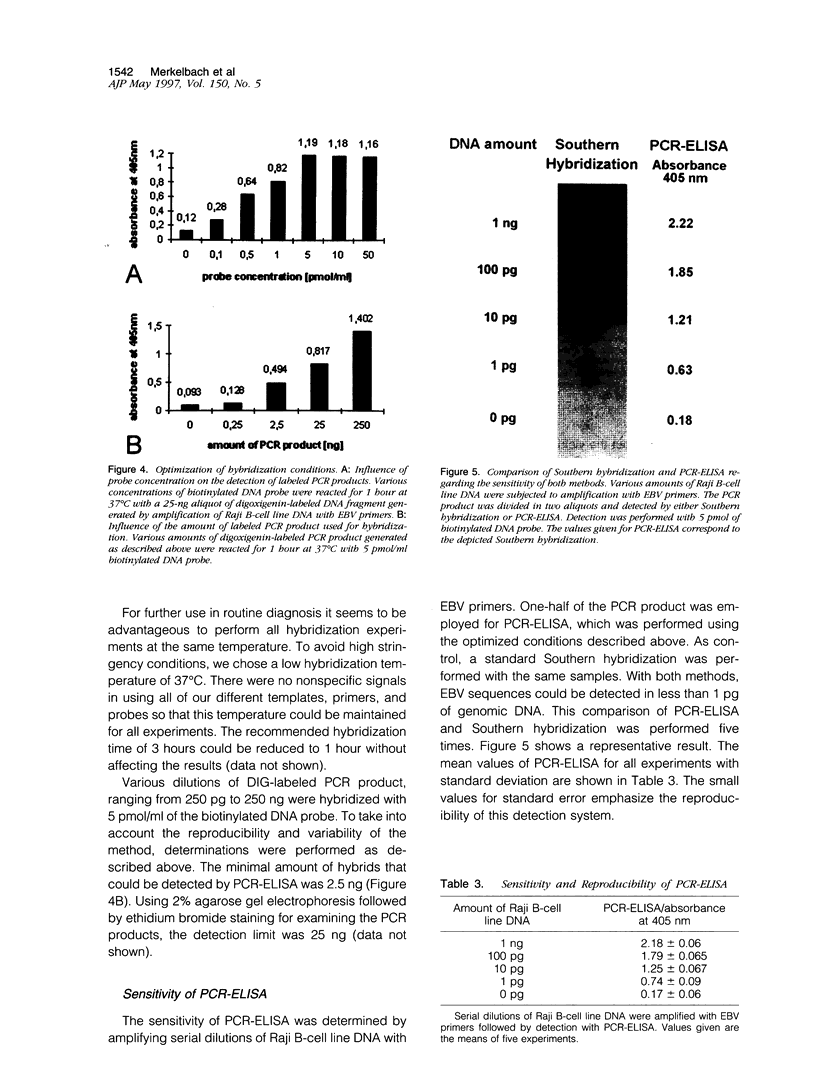
Four different DNA extraction methods were compared to determine their ability to provide DNA for amplification of viral sequences from paraffin-embedded human tissue samples by polymerase chain reaction (PCR). The suitability of extraction methods was assessed using parameters like DNA yield, length of recovered DNA fragments, and duration. Furthermore, the efficiency of amplifying a human single-copy gene, the beta-globin gene, from DNA samples was tested. The best preservation of DNA molecules could be achieved by binding the DNA onto a silica column before further purification. Viral DNA sequences could be amplified by PCR in DNA extracted from routinely processed paraffin blocks from cases with clinically or morphologically suspected cytomegalovirus or Epstein-Barr virus infections. The PCR products were specified by a novel liquid hybridization assay called PCR-enzyme-linked immunosorbent assay. Using this assay, the time-consuming Southern hybridization could be replaced and the time requirement for the detection of PCR products could be reduced from 1 day to 4 hours. The assay system described here represents a reliable, sensitive, and specific method for the detection of viral DNA from paraffin-embedded tissue samples.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An S. F., Ciardi A., Scaravilli F. PCR detection of HIV proviral DNA (gag) in the brains of patients with AIDS: comparison between results using fresh frozen and paraffin wax embedded specimens. J Clin Pathol. 1994 Nov;47(11):990–994. doi: 10.1136/jcp.47.11.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. K., Makdisi W. F., Weston A. P., Mitchell S. M., Campbell D. R. Microwave-based DNA extraction from paraffin-embedded tissue for PCR amplification. Biotechniques. 1995 May;18(5):768-70, 772-3. [PubMed] [Google Scholar]
- Bobo L., Coutlee F., Yolken R. H., Quinn T., Viscidi R. P. Diagnosis of Chlamydia trachomatis cervical infection by detection of amplified DNA with an enzyme immunoassay. J Clin Microbiol. 1990 Sep;28(9):1968–1973. doi: 10.1128/jcm.28.9.1968-1973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates P. J., d'Ardenne A. J., Khan G., Kangro H. O., Slavin G. Simplified procedures for applying the polymerase chain reaction to routinely fixed paraffin wax sections. J Clin Pathol. 1991 Feb;44(2):115–118. doi: 10.1136/jcp.44.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlee F., Viscidi R. P., Yolken R. H. Comparison of colorimetric, fluorescent, and enzymatic amplification substrate systems in an enzyme immunoassay for detection of DNA-RNA hybrids. J Clin Microbiol. 1989 May;27(5):1002–1007. doi: 10.1128/jcm.27.5.1002-1007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlée F., Bobo L., Abbass H., Dalabetta G., Hook N. E., Shah K., Viscidi R. P. Detection of HPV-16 in cell lines and cervical lavage specimens by a polymerase chain reaction-enzyme immunoassay assay. J Med Virol. 1992 May;37(1):22–29. doi: 10.1002/jmv.1890370105. [DOI] [PubMed] [Google Scholar]
- Dubeau L., Chandler L. A., Gralow J. R., Nichols P. W., Jones P. A. Southern blot analysis of DNA extracted from formalin-fixed pathology specimens. Cancer Res. 1986 Jun;46(6):2964–2969. [PubMed] [Google Scholar]
- Forsthoefel K. F., Papp A. C., Snyder P. J., Prior T. W. Optimization of DNA extraction from formalin-fixed tissue and its clinical application in Duchenne muscular dystrophy. Am J Clin Pathol. 1992 Jul;98(1):98–104. doi: 10.1093/ajcp/98.1.98. [DOI] [PubMed] [Google Scholar]
- Garweg J., Fenner T., Böhnke M., Schmitz H. An improved technique for the diagnosis of viral retinitis from samples of aqueous humor and vitreous. Graefes Arch Clin Exp Ophthalmol. 1993 Sep;231(9):508–513. doi: 10.1007/BF00921115. [DOI] [PubMed] [Google Scholar]
- Goelz S. E., Hamilton S. R., Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985 Jul 16;130(1):118–126. doi: 10.1016/0006-291x(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Keller G. H., Huang D. P., Manak M. M. Detection of human immunodeficiency virus type 1 DNA by polymerase chain reaction amplification and capture hybridization in microtiter wells. J Clin Microbiol. 1991 Mar;29(3):638–641. doi: 10.1128/jcm.29.3.638-641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene P., Milde-Langosch K., Runkel M., Schulz K., Löning T. A simple and rapid technique to process formalin-fixed, paraffin-embedded tissues for the detection of viruses by the polymerase chain reaction. Virchows Arch A Pathol Anat Histopathol. 1992;420(3):269–273. doi: 10.1007/BF01600280. [DOI] [PubMed] [Google Scholar]
- Landgraf A., Reckmann B., Pingoud A. Direct analysis of polymerase chain reaction products using enzyme-linked immunosorbent assay techniques. Anal Biochem. 1991 Oct;198(1):86–91. doi: 10.1016/0003-2697(91)90510-z. [DOI] [PubMed] [Google Scholar]
- Lazar J. G. Advanced methods in PCR product detection. PCR Methods Appl. 1994 Aug;4(1):S1–14. doi: 10.1101/gr.4.1.s1. [DOI] [PubMed] [Google Scholar]
- Lorenzen J., Jux G., Zhao-Höhn M., Klöckner A., Fischer R., Hansmann M. L. Detection of T-cell clonality in paraffin-embedded tissues. Diagn Mol Pathol. 1994 Jun;3(2):93–99. doi: 10.1097/00019606-199406000-00005. [DOI] [PubMed] [Google Scholar]
- Luk J. M. A PCR enzyme immunoassay for detection of Salmonella typhi. Biotechniques. 1994 Dec;17(6):1038-40, 1042. [PubMed] [Google Scholar]
- Lungu O., Sun X. W., Wright T. C., Jr, Ferenczy A., Richart R. M., Silverstein S. A polymerase chain reaction-enzyme-linked immunosorbent assay method for detecting human papillomavirus in cervical carcinomas and high-grade cervical cancer precursors. Obstet Gynecol. 1995 Mar;85(3):337–342. doi: 10.1016/0029-7844(94)00399-x. [DOI] [PubMed] [Google Scholar]
- Moul J. W., Bishoff J. T., Theune S. M., Chang E. H. Absent ras gene mutations in human adrenal cortical neoplasms and pheochromocytomas. J Urol. 1993 Jun;149(6):1389–1394. doi: 10.1016/s0022-5347(17)36397-8. [DOI] [PubMed] [Google Scholar]
- Park J. S., Leake J. F., Sharma B. K., Toki T., Kessis T. D., Ambros R. A., Shah K. V. Variability in beta-globin and HPV DNA amplification by PCR from fixed tissues. Mod Pathol. 1991 Sep;4(5):667–670. [PubMed] [Google Scholar]
- Peiper S. C., Myers J. L., Broussard E. E., Sixbey J. W. Detection of Epstein-Barr virus genomes in archival tissues by polymerase chain reaction. Arch Pathol Lab Med. 1990 Jul;114(7):711–714. [PubMed] [Google Scholar]
- Rogers B. B., Alpert L. C., Hine E. A., Buffone G. J. Analysis of DNA in fresh and fixed tissue by the polymerase chain reaction. Am J Pathol. 1990 Mar;136(3):541–548. [PMC free article] [PubMed] [Google Scholar]
- Sepp R., Szabó I., Uda H., Sakamoto H. Rapid techniques for DNA extraction from routinely processed archival tissue for use in PCR. J Clin Pathol. 1994 Apr;47(4):318–323. doi: 10.1136/jcp.47.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Raoult D. A simple method for amplification of DNA from paraffin-embedded tissues. Nucleic Acids Res. 1992 Oct 11;20(19):5237–5238. doi: 10.1093/nar/20.19.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. S., Metzger D. A., Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991 Apr;10(4):506–513. [PubMed] [Google Scholar]
- Whetsell A. J., Drew J. B., Milman G., Hoff R., Dragon E. A., Adler K., Hui J., Otto P., Gupta P., Farzadegan H. Comparison of three nonradioisotopic polymerase chain reaction-based methods for detection of human immunodeficiency virus type 1. J Clin Microbiol. 1992 Apr;30(4):845–853. doi: 10.1128/jcm.30.4.845-853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. F., Reid A. H., Tsai M. M., Ventre K. M., Murari P. J., Frizzera G., O'Leary T. J. Detection of Epstein-Barr virus sequences in Hodgkin's disease by the polymerase chain reaction. Am J Pathol. 1991 Aug;139(2):393–398. [PMC free article] [PubMed] [Google Scholar]
- Zaki S. R., Judd R., Coffield L. M., Greer P., Rolston F., Evatt B. L. Human papillomavirus infection and anal carcinoma. Retrospective analysis by in situ hybridization and the polymerase chain reaction. Am J Pathol. 1992 Jun;140(6):1345–1355. [PMC free article] [PubMed] [Google Scholar]
- de Franchis R., Cross N. C., Foulkes N. S., Cox T. M. A potent inhibitor of Taq polymerase copurifies with human genomic DNA. Nucleic Acids Res. 1988 Nov 11;16(21):10355–10355. doi: 10.1093/nar/16.21.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]






