Abstract
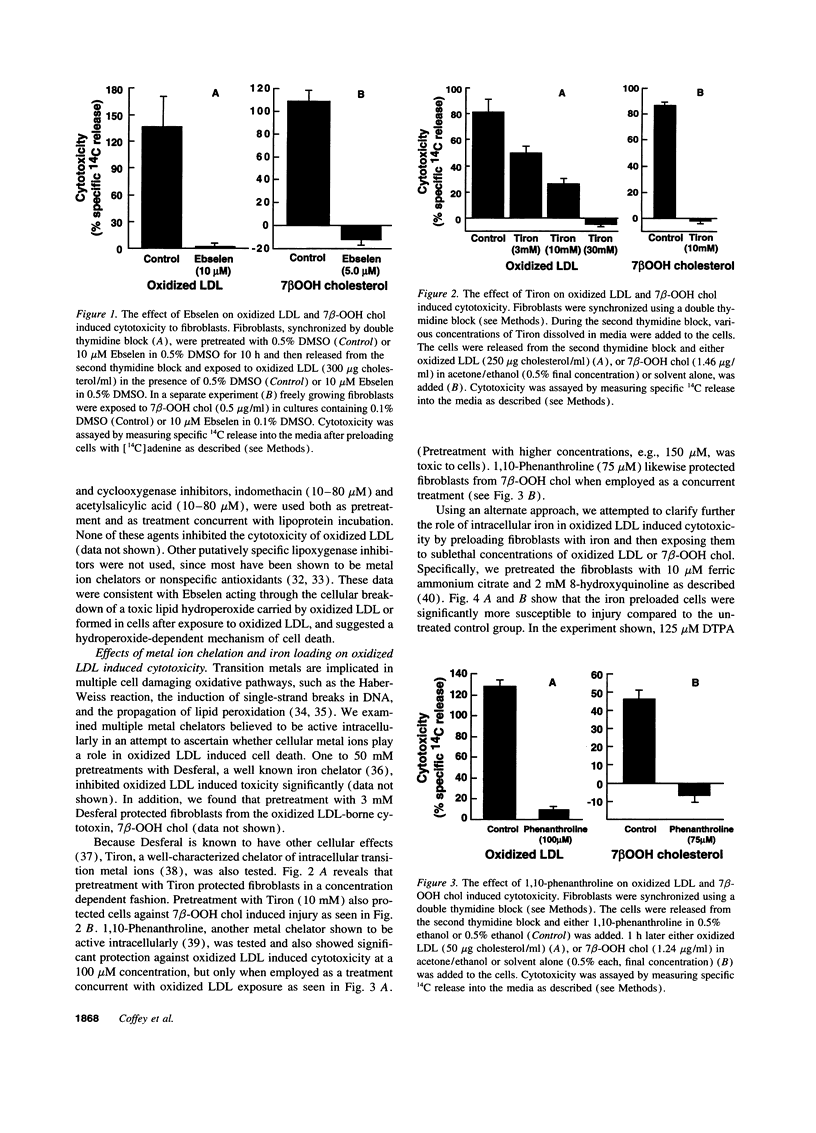
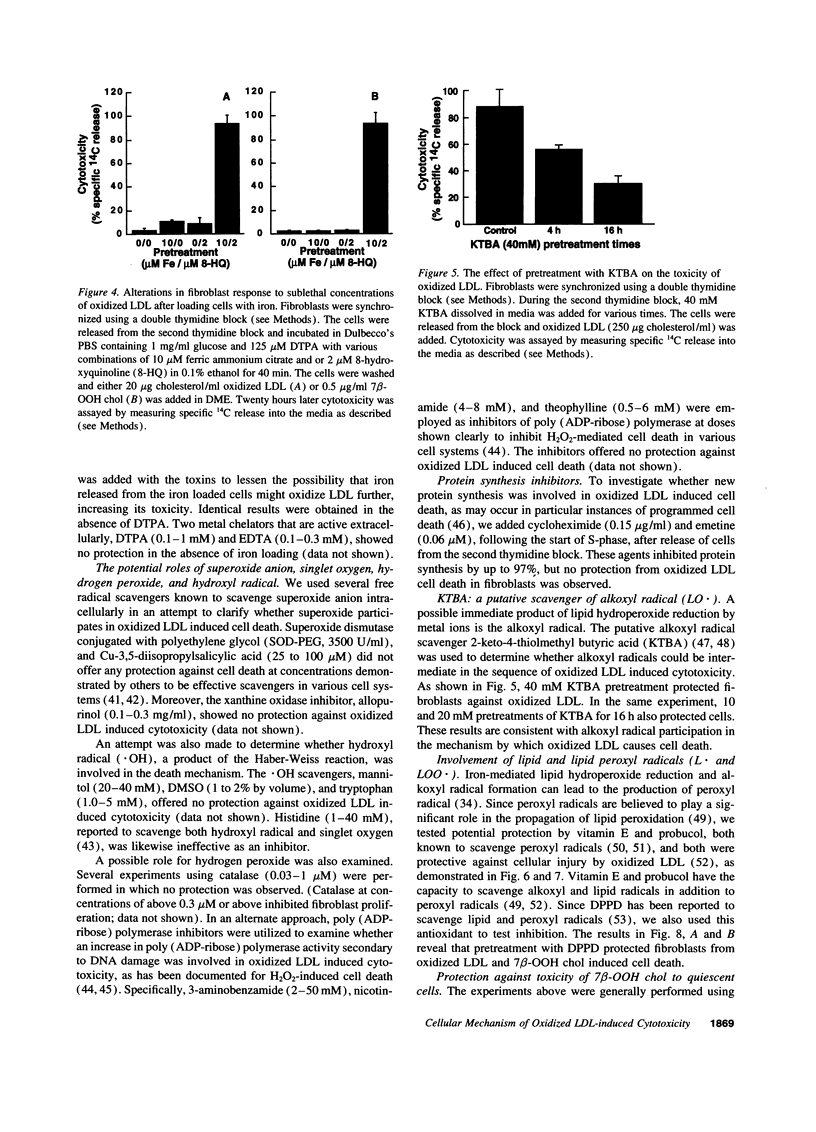
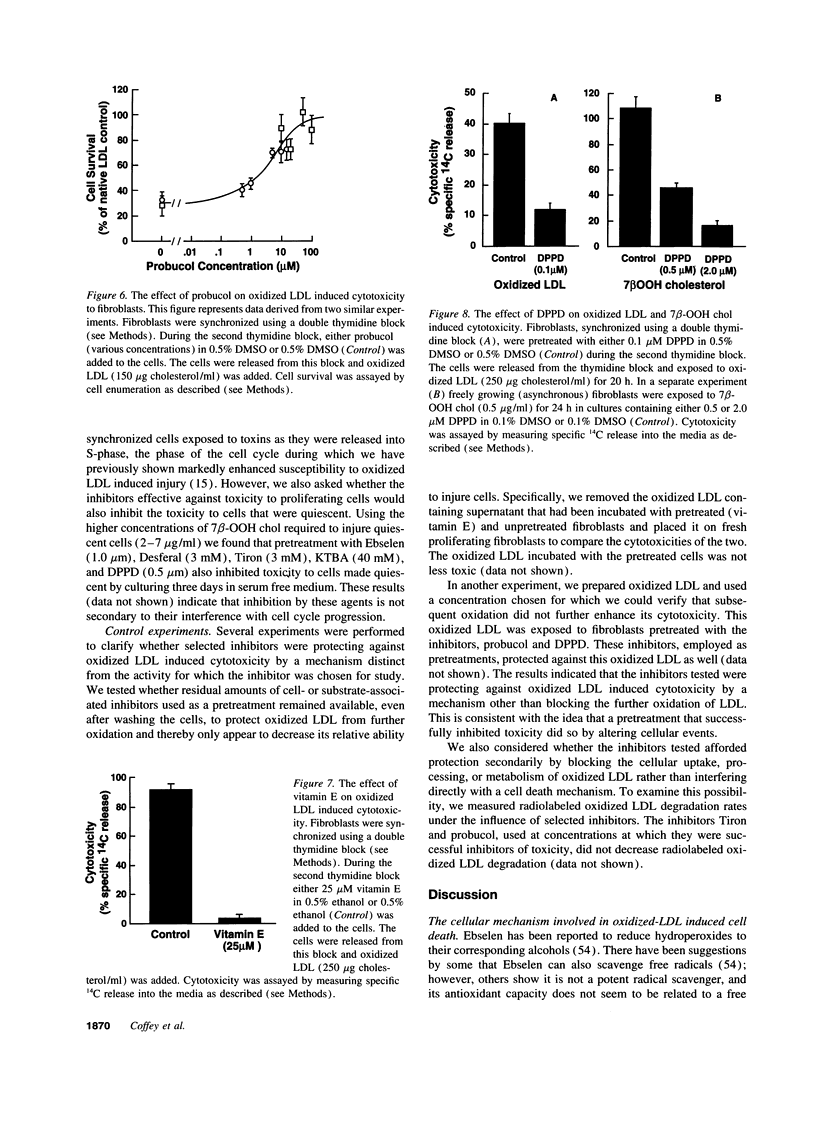
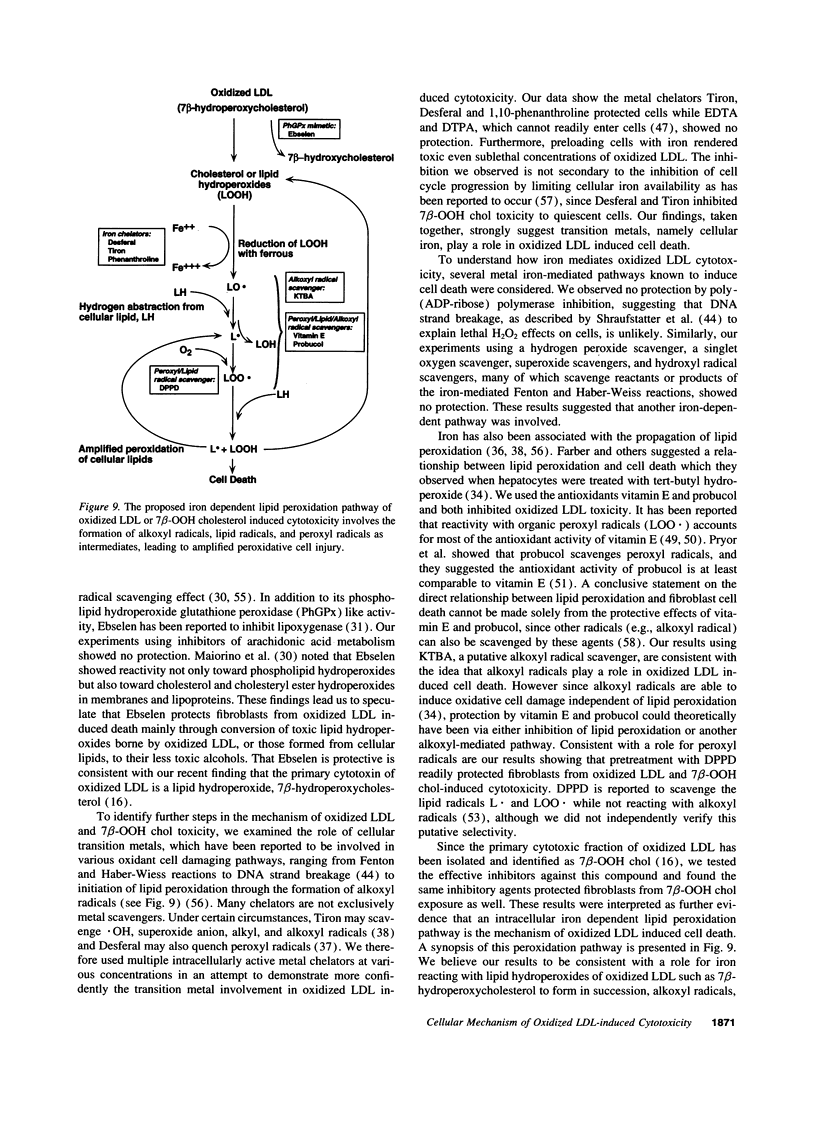
Mounting evidence supports current theories linking lipoprotein oxidation to atherosclerosis. We sought the cellular biochemical mechanism by which oxidized LDL inflicts cell injury. Inhibitors of candidate pathways of cell death were used to treat human fibroblast target cells exposed to oxidized LDL.. Ebselen, which degrades lipid hydroperoxides, inhibited oxidized LDL toxicity, consistent with our recent report that 7 beta-hydroperoxycholesterol (7 beta-OOH chol) is the major cytotoxin of oxidized LDL. Intracellular chelation of metal ions inhibited, while preloading cells with iron enhanced, toxicity, Inhibition of oxidized LDL and 7 beta-OOH chol toxicity by 2-keto-4-thiolmethyl butyric acid, a putative alkoxyl radical scavenger and by vitamin E, probucol and diphenylphenylenediamine, putative scavengers of peroxyl radicals was consistent with the involvement of these radicals in the lethal sequence. Cell death was thus postulated to occur due to lipid peroxidation via a sequence involving lipid hydroperoxide-induced, iron-mediated formation of alkoxyl, lipid, and peroxyl radicals. Pathways involving other reactive oxygen species, new protein synthesis, or altered cholesterol metabolism were considered less likely, since putative inhibitors failed to lessen toxicity. Understanding the mechanism of cell injury by oxidized LDL and its toxic moiety, 7 beta-OOH chol, may indicate specific interventions in the cell injury believed to accompany vascular lesion development.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balla G., Vercellotti G. M., Eaton J. W., Jacob H. S. Iron loading of endothelial cells augments oxidant damage. J Lab Clin Med. 1990 Oct;116(4):546–554. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974 Nov 25;249(22):7306–7314. [PubMed] [Google Scholar]
- Børsum T., Henriksen T., Carlander B., Reisvaag A. Injury to human cells in culture induced by low density lipoprotein: an effect independent of receptor binding and endocytotic uptake of low density lipoprotein. Scand J Clin Lab Invest. 1982 Feb;42(1):75–81. [PubMed] [Google Scholar]
- Chisolm G. M., 3rd, Sila C. A., Hmiel S. P. Measurements of the degradation products of radioiodinated proteins. Anal Biochem. 1981 Mar 1;111(2):212–219. doi: 10.1016/0003-2697(81)90556-x. [DOI] [PubMed] [Google Scholar]
- Chisolm G. M., Ma G., Irwin K. C., Martin L. L., Gunderson K. G., Linberg L. F., Morel D. W., DiCorleto P. E. 7 beta-hydroperoxycholest-5-en-3 beta-ol, a component of human atherosclerotic lesions, is the primary cytotoxin of oxidized human low density lipoprotein. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11452–11456. doi: 10.1073/pnas.91.24.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix T. A., Aikens J. Mechanisms and biological relevance of lipid peroxidation initiation. Chem Res Toxicol. 1993 Jan-Feb;6(1):2–18. doi: 10.1021/tx00031a001. [DOI] [PubMed] [Google Scholar]
- Dousset N., Negre-Salvayre A., Lopez M., Salvayre R., Douste-Blazy L. Ultraviolet-treated lipoproteins as a model system for the study of the biological effects of lipid peroxides on cultured cell. I. Chemical modifications of ultraviolet-treated low-density lipoproteins. Biochim Biophys Acta. 1990 Aug 6;1045(3):219–223. doi: 10.1016/0005-2760(90)90123-f. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Kyle M. E., Coleman J. B. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990 Jun;62(6):670–679. [PubMed] [Google Scholar]
- Fox P. L., Chisolm G. M., DiCorleto P. E. Lipoprotein-mediated inhibition of endothelial cell production of platelet-derived growth factor-like protein depends on free radical lipid peroxidation. J Biol Chem. 1987 May 5;262(13):6046–6054. [PubMed] [Google Scholar]
- Freeman M., Ekkel Y., Rohrer L., Penman M., Freedman N. J., Chisolm G. M., Krieger M. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4931–4935. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Ma G. P., Chisolm G. M. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. J Immunol. 1990 Mar 15;144(6):2343–2350. [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Henriksen T., Evensen S. A., Carlander B. Injury to cultured endothelial cells induced by low density lipoproteins: protection by high density lipoproteins. Scand J Clin Lab Invest. 1979 Jun;39(4):369–375. doi: 10.3109/00365517909106121. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Evensen S. A., Carlander B. Injury to human endothelial cells in culture induced by low density lipoproteins. Scand J Clin Lab Invest. 1979 Jun;39(4):361–368. doi: 10.3109/00365517909106120. [DOI] [PubMed] [Google Scholar]
- Hessler J. R., Morel D. W., Lewis L. J., Chisolm G. M. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983 May-Jun;3(3):215–222. doi: 10.1161/01.atv.3.3.215. [DOI] [PubMed] [Google Scholar]
- Hessler J. R., Robertson A. L., Jr, Chisolm G. M., 3rd LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis. 1979 Mar;32(3):213–229. doi: 10.1016/0021-9150(79)90166-7. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Yajima N., Yamaguchi N., Ishida M., Katoh Y., Harada T., Terano A., Ivey K. J. Antioxidant protection against oxidant-induced damage in cultured gastric mucosal cells. Gastroenterol Jpn. 1993 May;28 (Suppl 5):132–138. doi: 10.1007/BF02989224. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., Gaubatz J. W. Isolation, purification, and characterization of a lipoprotein containing Apo B from the human aorta. Atherosclerosis. 1982 Apr;42(2-3):273–297. doi: 10.1016/0021-9150(82)90157-5. [DOI] [PubMed] [Google Scholar]
- Hughes H., Mathews B., Lenz M. L., Guyton J. R. Cytotoxicity of oxidized LDL to porcine aortic smooth muscle cells is associated with the oxysterols 7-ketocholesterol and 7-hydroxycholesterol. Arterioscler Thromb. 1994 Jul;14(7):1177–1185. doi: 10.1161/01.atv.14.7.1177. [DOI] [PubMed] [Google Scholar]
- Jürgens G., Hoff H. F., Chisolm G. M., 3rd, Esterbauer H. Modification of human serum low density lipoprotein by oxidation--characterization and pathophysiological implications. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):315–336. doi: 10.1016/0009-3084(87)90070-3. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Kosugi K., Morel D. W., DiCorleto P. E., Chisolm G. M. Toxicity of oxidized low-density lipoprotein to cultured fibroblasts is selective for S phase of the cell cycle. J Cell Physiol. 1987 Mar;130(3):311–320. doi: 10.1002/jcp.1041300302. [DOI] [PubMed] [Google Scholar]
- Krishna C. M., Liebmann J. E., Kaufman D., DeGraff W., Hahn S. M., McMurry T., Mitchell J. B., Russo A. The catecholic metal sequestering agent 1,2-dihydroxybenzene-3,5-disulfonate confers protection against oxidative cell damage. Arch Biochem Biophys. 1992 Apr;294(1):98–106. doi: 10.1016/0003-9861(92)90142-j. [DOI] [PubMed] [Google Scholar]
- Kukreja R. C., Loesser K. E., Kearns A. A., Naseem S. A., Hess M. L. Protective effects of histidine during ischemia-reperfusion in isolated perfused rat hearts. Am J Physiol. 1993 May;264(5 Pt 2):H1370–H1381. doi: 10.1152/ajpheart.1993.264.5.H1370. [DOI] [PubMed] [Google Scholar]
- Kuzuya M., Naito M., Funaki C., Hayashi T., Asai K., Kuzuya F. Probucol prevents oxidative injury to endothelial cells. J Lipid Res. 1991 Feb;32(2):197–204. [PubMed] [Google Scholar]
- Kuzuya M., Naito M., Yamada K., Funaki C., Hayashi T., Asai K., Kuzuya F. Involvement of intracellular iron in the toxicity of oxidized low density lipoprotein to cultured endothelial cells. Biochem Int. 1990 Nov;22(3):567–573. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leuthauser S. W., Oberley L. W., Oberley T. D., Sorenson J. R., Ramakrishna K. Antitumor effect of a copper coordination compound with superoxide dismutase-like activity. J Natl Cancer Inst. 1981 Jun;66(6):1077–1081. doi: 10.1093/jnci/66.6.1077. [DOI] [PubMed] [Google Scholar]
- Liebler D. C. The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol. 1993;23(2):147–169. doi: 10.3109/10408449309117115. [DOI] [PubMed] [Google Scholar]
- Maiorino M., Roveri A., Ursini F. Antioxidant effect of Ebselen (PZ 51): peroxidase mimetic activity on phospholipid and cholesterol hydroperoxides vs free radical scavenger activity. Arch Biochem Biophys. 1992 Jun;295(2):404–409. doi: 10.1016/0003-9861(92)90534-4. [DOI] [PubMed] [Google Scholar]
- Masaki N., Kyle M. E., Farber J. L. tert-butyl hydroperoxide kills cultured hepatocytes by peroxidizing membrane lipids. Arch Biochem Biophys. 1989 Mar;269(2):390–399. doi: 10.1016/0003-9861(89)90122-7. [DOI] [PubMed] [Google Scholar]
- McNally A. K., Chisolm G. M., 3rd, Morel D. W., Cathcart M. K. Activated human monocytes oxidize low-density lipoprotein by a lipoxygenase-dependent pathway. J Immunol. 1990 Jul 1;145(1):254–259. [PubMed] [Google Scholar]
- Minotti G. Sources and role of iron in lipid peroxidation. Chem Res Toxicol. 1993 Mar-Apr;6(2):134–146. doi: 10.1021/tx00032a001. [DOI] [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Morel I., Cillard J., Lescoat G., Sergent O., Pasdeloup N., Ocaktan A. Z., Abdallah M. A., Brissot P., Cillard P. Antioxidant and free radical scavenging activities of the iron chelators pyoverdin and hydroxypyrid-4-ones in iron-loaded hepatocyte cultures: comparison of their mechanism of protection with that of desferrioxamine. Free Radic Biol Med. 1992 Nov;13(5):499–508. doi: 10.1016/0891-5849(92)90144-6. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A., Alomar Y., Troly M., Salvayre R. Ultraviolet-treated lipoproteins as a model system for the study of the biological effects of lipid peroxides on cultured cells. III. The protective effect of antioxidants (probucol, catechin, vitamin E) against the cytotoxicity of oxidized LDL occurs in two different ways. Biochim Biophys Acta. 1991 Jun 5;1096(4):291–300. doi: 10.1016/0925-4439(91)90065-h. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A., Lopez M., Levade T., Pieraggi M. T., Dousset N., Douste-Blazy L., Salvayre R. Ultraviolet-treated lipoproteins as a model system for the study of the biological effects of lipid peroxides on cultured cells. II. Uptake and cytotoxicity of ultraviolet-treated LDL on lymphoid cell lines. Biochim Biophys Acta. 1990 Aug 6;1045(3):224–232. doi: 10.1016/0005-2760(90)90124-g. [DOI] [PubMed] [Google Scholar]
- Noguchi N., Yoshida Y., Kaneda H., Yamamoto Y., Niki E. Action of ebselen as an antioxidant against lipid peroxidation. Biochem Pharmacol. 1992 Jul 7;44(1):39–44. doi: 10.1016/0006-2952(92)90035-h. [DOI] [PubMed] [Google Scholar]
- Nègre-Salvayre A., Salvayre R. Quercetin prevents the cytotoxicity of oxidized LDL on lymphoid cell lines. Free Radic Biol Med. 1992;12(2):101–106. doi: 10.1016/0891-5849(92)90002-x. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Wieland E., Steinberg D. A role for endothelial cell lipoxygenase in the oxidative modification of low density lipoprotein. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1046–1050. doi: 10.1073/pnas.86.3.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn M. S., Chisolm G. M. Oxidized lipoproteins, altered cell function and atherosclerosis. Atherosclerosis. 1994 Aug;108 (Suppl):S21–S29. doi: 10.1016/0021-9150(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Roussillon S., Astruc M., Defay R., Tabacik C., Descomps B., Crastes de Paulet A. DNA and cholesterol biosynthesis in synchronized embryonic rat fibroblasts. I. Temporal relationships between HMG-CoA reductase activity, sterol biosynthesis and thymidine incorporation into DNA. Biochim Biophys Acta. 1983 Aug 17;763(1):1–10. doi: 10.1016/0167-4889(83)90018-6. [DOI] [PubMed] [Google Scholar]
- Safayhi H., Tiegs G., Wendel A. A novel biologically active seleno-organic compound--V. Inhibition by ebselen (PZ 51) of rat peritoneal neutrophil lipoxygenase. Biochem Pharmacol. 1985 Aug 1;34(15):2691–2694. doi: 10.1016/0006-2952(85)90569-6. [DOI] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Hinshaw D. B., Spragg R. G., Sklar L. A., Cochrane C. G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S. Programmed cell death: concept, mechanism and control. Biol Rev Camb Philos Soc. 1992 Aug;67(3):287–319. doi: 10.1111/j.1469-185x.1992.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Shirhatti V., Krishna G. A simple and sensitive method for monitoring drug-induced cell injury in cultured cells. Anal Biochem. 1985 Jun;147(2):410–418. doi: 10.1016/0003-2697(85)90290-8. [DOI] [PubMed] [Google Scholar]
- Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med. 1993 Mar;14(3):313–323. doi: 10.1016/0891-5849(93)90028-s. [DOI] [PubMed] [Google Scholar]
- Sies H., Stahl W., Sundquist A. R. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci. 1992 Sep 30;669:7–20. doi: 10.1111/j.1749-6632.1992.tb17085.x. [DOI] [PubMed] [Google Scholar]
- Smith E. B., Slater R. S. Relationship between low-density lipoprotein in aortic intima and serum-lipid levels. Lancet. 1972 Feb 26;1(7748):463–469. doi: 10.1016/s0140-6736(72)90122-5. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Thomas J. P., Geiger P. G., Girotti A. W. Lethal damage to endothelial cells by oxidized low density lipoprotein: role of selenoperoxidases in cytoprotection against lipid hydroperoxide- and iron-mediated reactions. J Lipid Res. 1993 Mar;34(3):479–490. [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Cholesterol metabolism in the macrophage. 3. Ingestion and intracellular fate of cholesterol and cholesterol esters. J Exp Med. 1972 Jan;135(1):21–44. doi: 10.1084/jem.135.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston G. W., Harvey W., Berl L., Cederbaum A. I. The generation of hydroxyl and alkoxyl radicals from the interaction of ferrous bipyridyl with peroxides. Biochem J. 1983 Nov 15;216(2):415–421. doi: 10.1042/bj2160415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Tsukidate K., Farber J. L. Differing effects of the inhibition of poly(ADP-ribose) polymerase on the course of oxidative cell injury in hepatocytes and fibroblasts. Biochem Pharmacol. 1993 Aug 3;46(3):483–491. doi: 10.1016/0006-2952(93)90525-2. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Steinberg D., Witztum J. L. Lipoproteins in normal and atherosclerotic aorta. Eur Heart J. 1990 Aug;11 (Suppl E):88–99. doi: 10.1093/eurheartj/11.suppl_e.88. [DOI] [PubMed] [Google Scholar]


