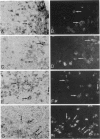
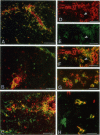
Abstract
Experimental autoimmune encephalomyelitis of the Lewis rat is a T-cell-mediated autoimmune disease of the central nervous system characterized by a self-limiting monophasic course. In this study, we analyzed the expression of the anti-inflammatory cytokine interleukin (IL)-10 at the mRNA and protein level in experimental autoimmune encephalomyelitis actively induced with the encephalitogenic 68-86 peptide of guinea pig myelin basic protein. Semiquantitative reverse transcriptase-polymerase chain reaction revealed that IL-10 mRNA expression peaked during the acute phase of the disease at days 11 and 13. IL-10 mRNA was synchronously induced with mRNA for the proinflammatory cytokine interferon-gamma. Immunocytochemistry with a monoclonal antibody against rat IL-10 showed that the peak of IL-10 mRNA was accompanied by an abundant expression of IL-10 protein during the acute stage of the disease. Both in situ hybridization and double labeling immunocytochemistry in combination with confocal microscopy identified T cells, macrophages/microglia, and astrocytes as major cellular sources of IL-10 in vivo. The early peak of IL-10 production was unexpected in light of its well-documented anti-inflammatory properties. Additional studies are required to determine whether endogenous IL-10 contributes to rapid clinical remission typical for Lewis rat experimental autoimmune encephalomyelitis or if it plays other, yet undefined, roles in central nervous system autoimmunity.
Full text
PDF


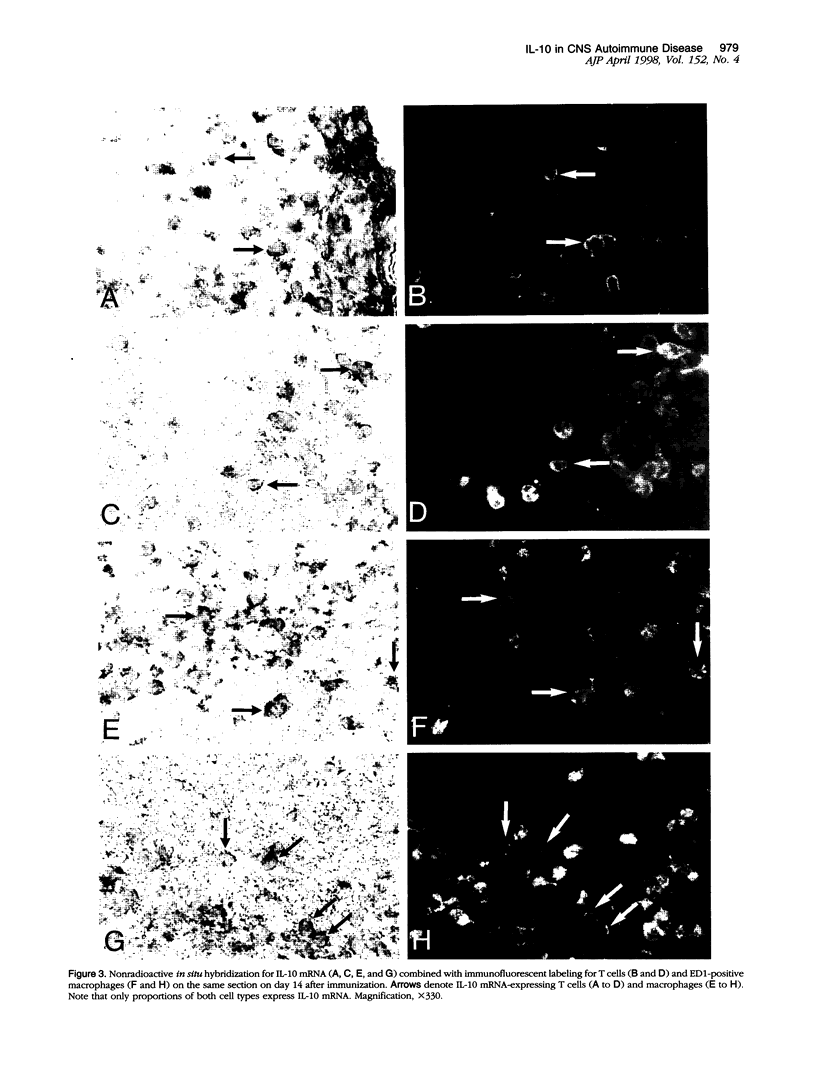
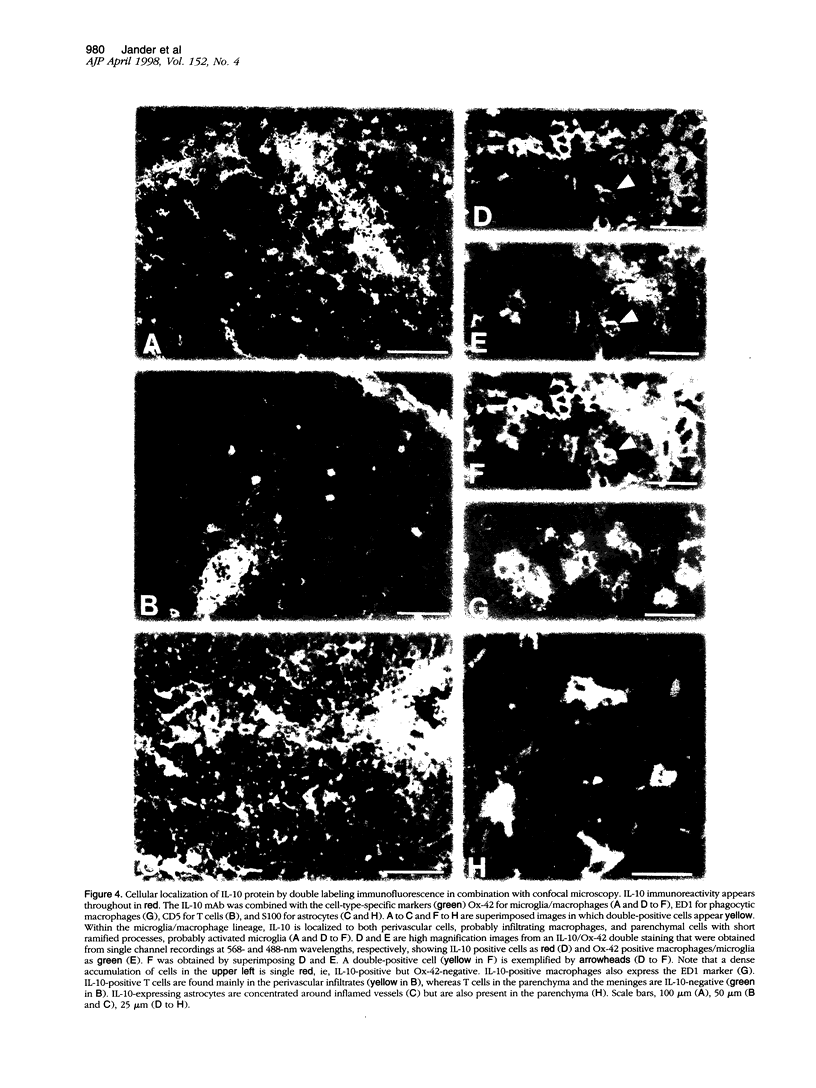


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Murphy K. M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996 Oct 31;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Bai X. F., Zhu J., Zhang G. X., Kaponides G., Höjeberg B., van der Meide P. H., Link H. IL-10 suppresses experimental autoimmune neuritis and down-regulates TH1-type immune responses. Clin Immunol Immunopathol. 1997 May;83(2):117–126. doi: 10.1006/clin.1997.4331. [DOI] [PubMed] [Google Scholar]
- Balasa B., Sarvetnick N. The paradoxical effects of interleukin 10 in the immunoregulation of autoimmune diabetes. J Autoimmun. 1996 Apr;9(2):283–286. doi: 10.1006/jaut.1996.0036. [DOI] [PubMed] [Google Scholar]
- Cannella B., Gao Y. L., Brosnan C., Raine C. S. IL-10 fails to abrogate experimental autoimmune encephalomyelitis. J Neurosci Res. 1996 Sep 15;45(6):735–746. doi: 10.1002/(SICI)1097-4547(19960915)45:6<735::AID-JNR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Cannella B., Raine C. S. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995 Apr;37(4):424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- Ding L., Linsley P. S., Huang L. Y., Germain R. N., Shevach E. M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993 Aug 1;151(3):1224–1234. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A. L., Foulcher E., Lemckert F. A., Sedgwick J. D. Microglia induce CD4 T lymphocyte final effector function and death. J Exp Med. 1996 Nov 1;184(5):1737–1745. doi: 10.1084/jem.184.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genain C. P., Abel K., Belmar N., Villinger F., Rosenberg D. P., Linington C., Raine C. S., Hauser S. L. Late complications of immune deviation therapy in a nonhuman primate. Science. 1996 Dec 20;274(5295):2054–2057. doi: 10.1126/science.274.5295.2054. [DOI] [PubMed] [Google Scholar]
- Goodman R. E., Oblak J., Bell R. G. Synthesis and characterization of rat interleukin-10 (IL-10) cDNA clones from the RNA of cultured OX8- OX22- thoracic duct T cells. Biochem Biophys Res Commun. 1992 Nov 30;189(1):1–7. doi: 10.1016/0006-291x(92)91516-s. [DOI] [PubMed] [Google Scholar]
- Issazadeh S., Ljungdahl A., Höjeberg B., Mustafa M., Olsson T. Cytokine production in the central nervous system of Lewis rats with experimental autoimmune encephalomyelitis: dynamics of mRNA expression for interleukin-10, interleukin-12, cytolysin, tumor necrosis factor alpha and tumor necrosis factor beta. J Neuroimmunol. 1995 Sep;61(2):205–212. doi: 10.1016/0165-5728(95)00100-g. [DOI] [PubMed] [Google Scholar]
- Jander S., Pohl J., Gillen C., Stoll G. Differential expression of interleukin-10 mRNA in Wallerian degeneration and immune-mediated inflammation of the rat peripheral nervous system. J Neurosci Res. 1996 Jan 15;43(2):254–259. doi: 10.1002/(SICI)1097-4547(19960115)43:2<254::AID-JNR13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kennedy M. K., Torrance D. S., Picha K. S., Mohler K. M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992 Oct 1;149(7):2496–2505. [PubMed] [Google Scholar]
- Matsumoto Y., Hanawa H., Tsuchida M., Abo T. In situ inactivation of infiltrating T cells in the central nervous system with autoimmune encephalomyelitis. The role of astrocytes. Immunology. 1993 Jul;79(3):381–390. [PMC free article] [PubMed] [Google Scholar]
- Meinl E., Aloisi F., Ertl B., Weber F., de Waal Malefyt R., Wekerle H., Hohlfeld R. Multiple sclerosis. Immunomodulatory effects of human astrocytes on T cells. Brain. 1994 Dec;117(Pt 6):1323–1332. doi: 10.1093/brain/117.6.1323. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Sawada M., Marunouchi T., Suzumura A. Production of interleukin-10 by mouse glial cells in culture. Biochem Biophys Res Commun. 1994 Dec 30;205(3):1907–1915. doi: 10.1006/bbrc.1994.2893. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F., McFarlin D. E., Raine C. S. Adoptive transfer of myelin basic protein-sensitized T cells produces chronic relapsing demyelinating disease in mice. Nature. 1984 May 24;309(5966):356–358. doi: 10.1038/309356a0. [DOI] [PubMed] [Google Scholar]
- Raine C. S. Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest. 1984 Jun;50(6):608–635. [PubMed] [Google Scholar]
- Rott O., Fleischer B., Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. Eur J Immunol. 1994 Jun;24(6):1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- Stoll G., Müller S., Schmidt B., van der Meide P., Jung S., Toyka K. V., Hartung H. P. Localization of interferon-gamma and Ia-antigen in T cell line-mediated experimental autoimmune encephalomyelitis. Am J Pathol. 1993 Jun;142(6):1866–1875. [PMC free article] [PubMed] [Google Scholar]
- Swanborg R. H. Experimental allergic encephalomyelitis. Methods Enzymol. 1988;162:413–421. doi: 10.1016/0076-6879(88)62095-7. [DOI] [PubMed] [Google Scholar]
- Wekerle H. Experimental autoimmune encephalomyelitis as a model of immune-mediated CNS disease. Curr Opin Neurobiol. 1993 Oct;3(5):779–784. doi: 10.1016/0959-4388(93)90153-p. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]