Abstract
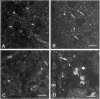
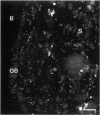
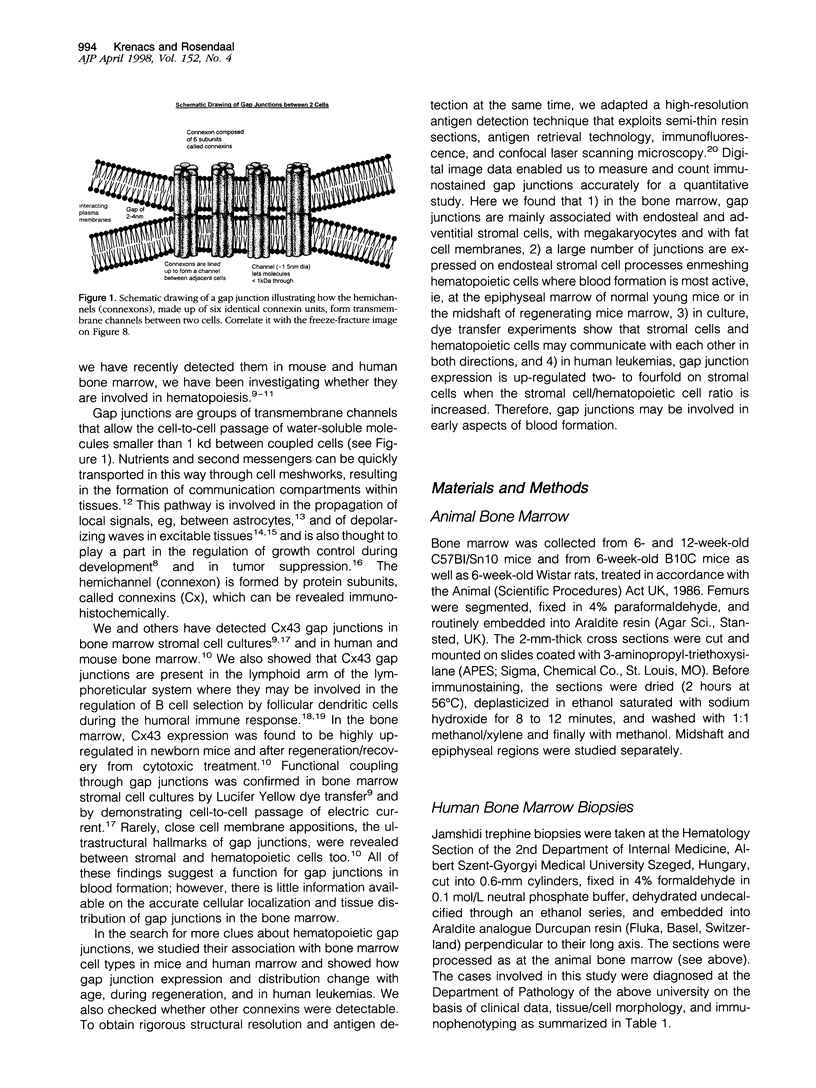
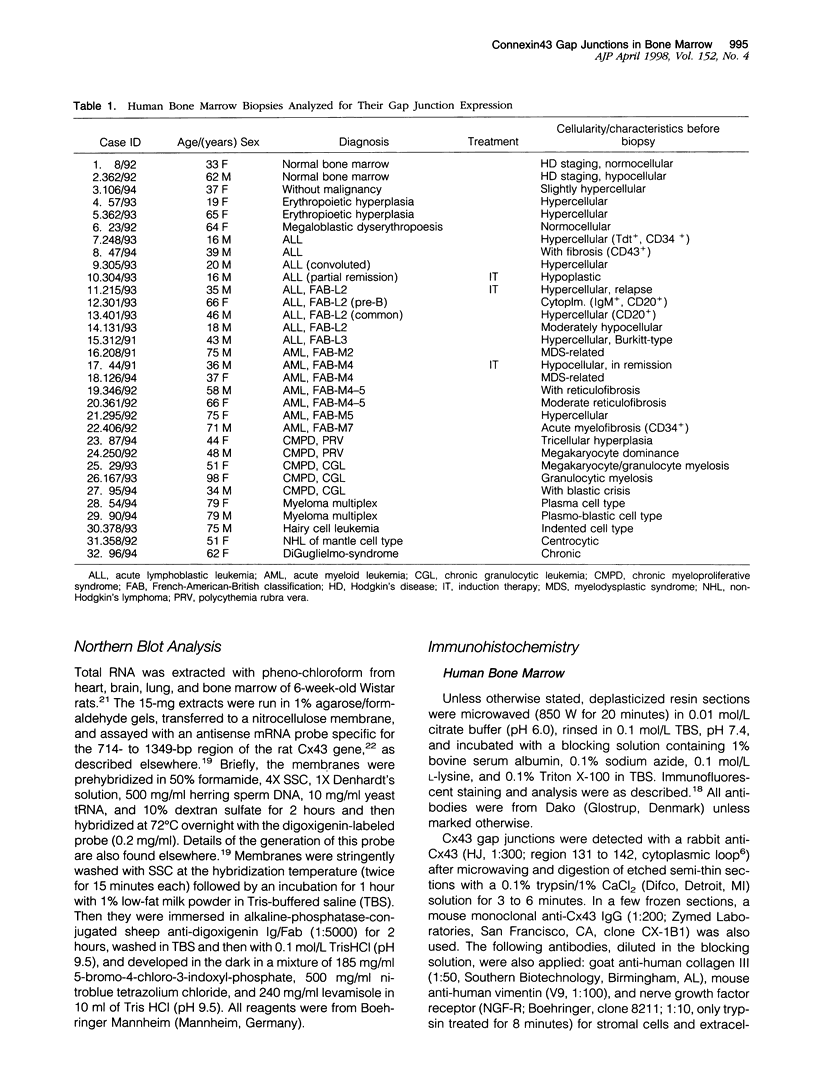
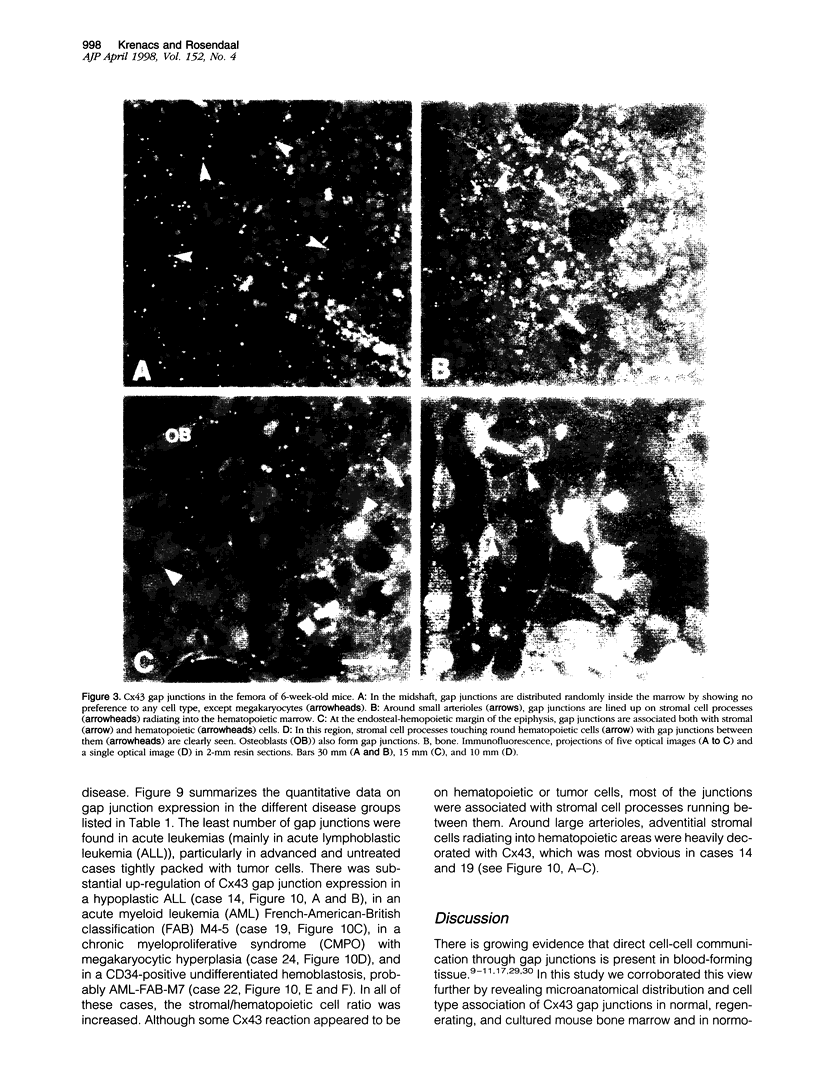
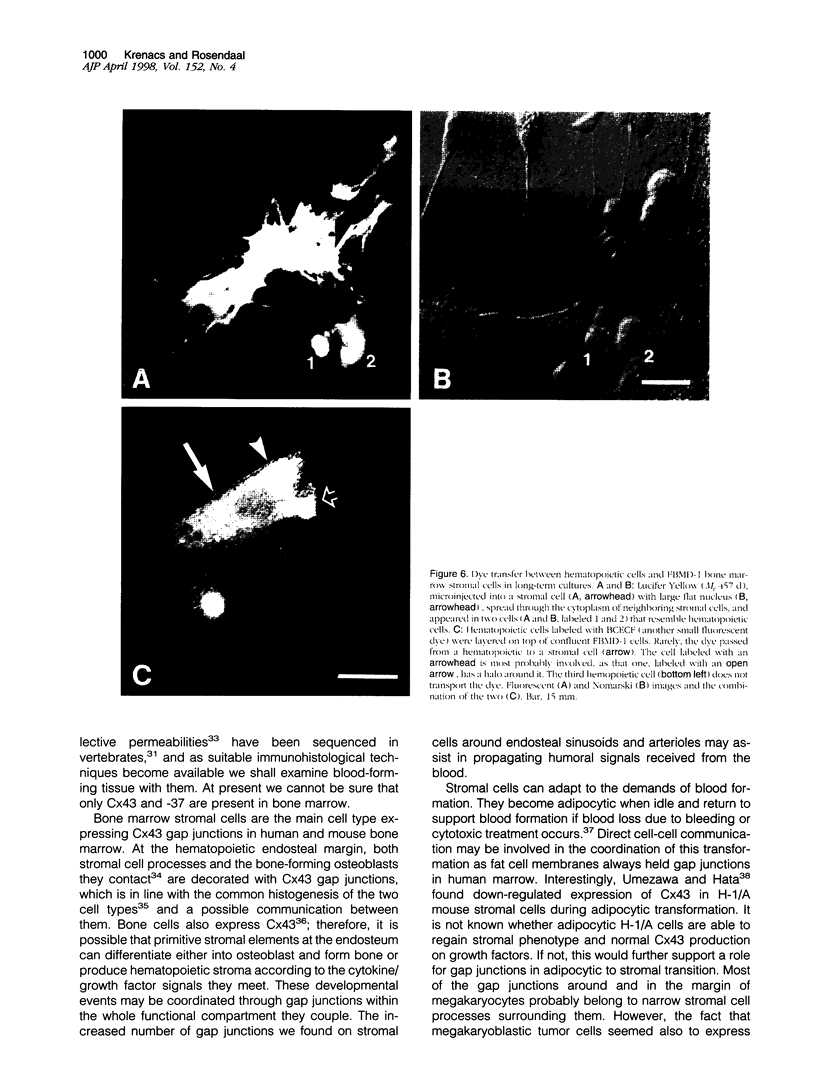
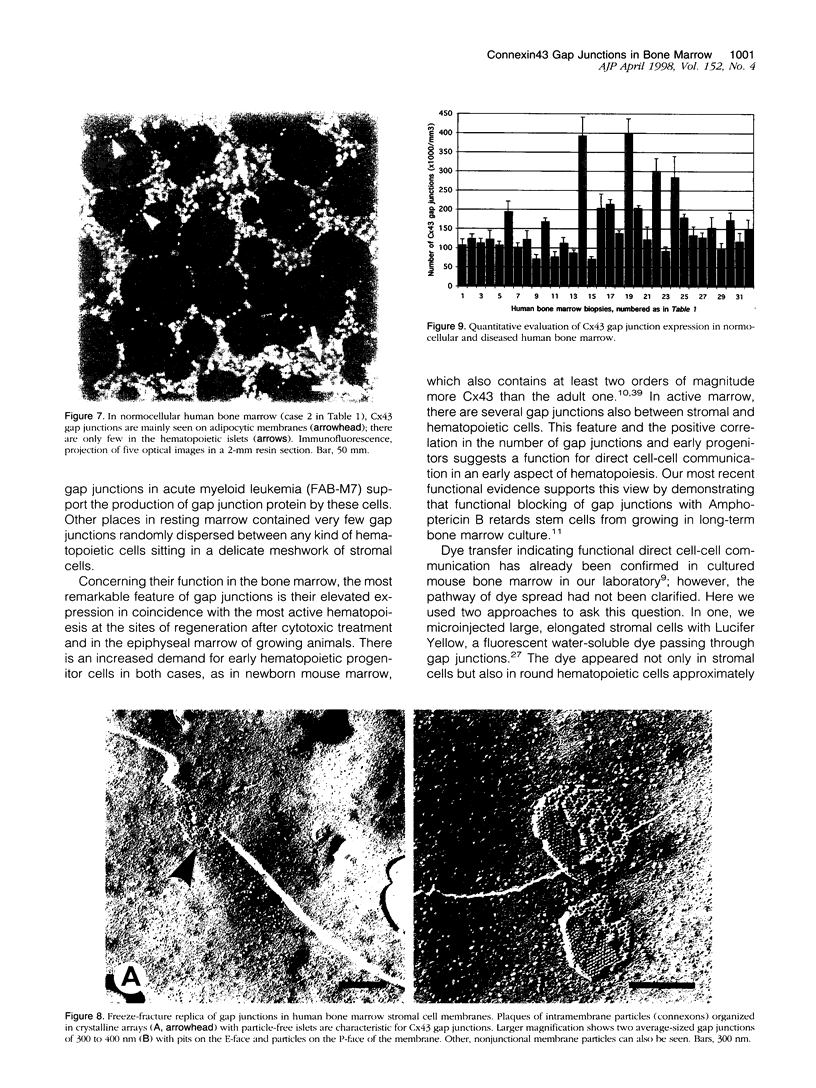
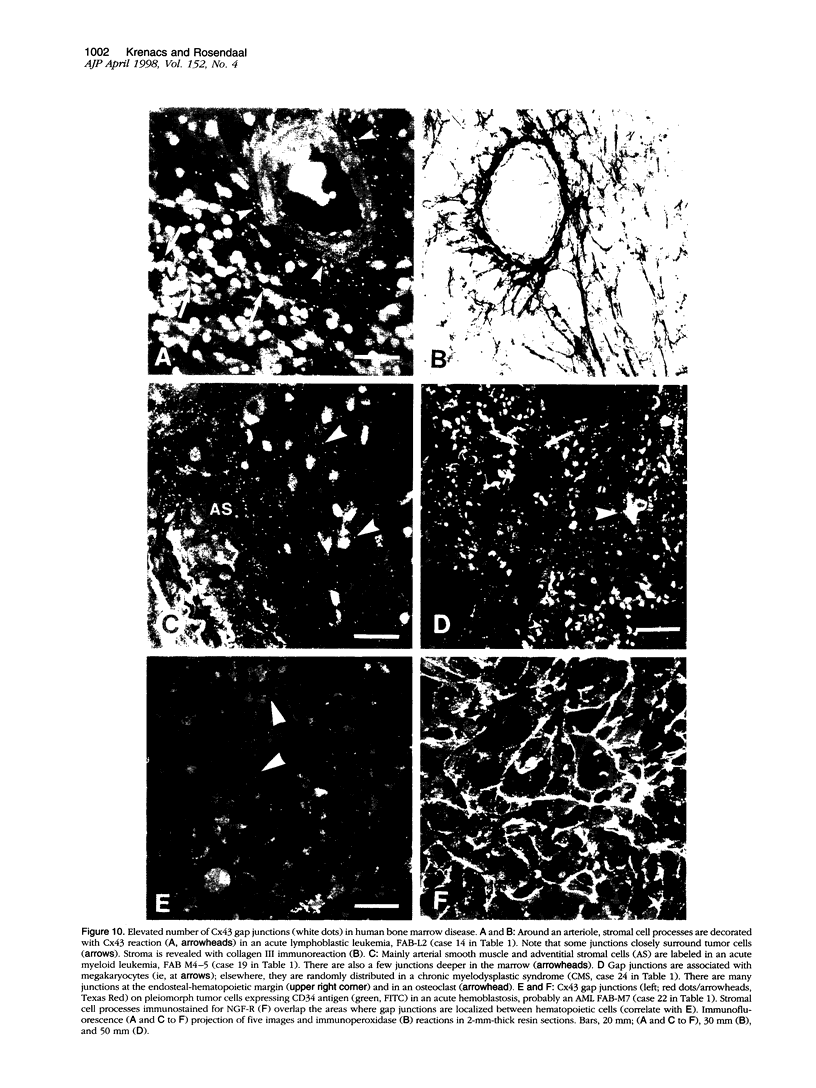
Communicating channels called gap junctions are thought to play a ubiquitous part in cell growth and development. Based on earlier work, we have recently found functional evidence of their presence in human and mouse bone marrow. In this study we studied the cell-type association of the gap junction channel-forming protein, connexin, in mouse and human bone marrow under different physiological and pathological conditions and tested the pathway of communication in bone marrow cultures. For high-resolution antigen demonstration we took advantage of semi-thin resin sections, antigen retrieval methods, immunofluorescence, and confocal laser scanning microscopy. Connexin43 (Cx43) and its mRNA were consistently expressed in human and rodent marrow. Cx37 was found only in the arteriolar endothelium, but neither Cx32 nor -26 were expressed. In tissue sections, the immunostained junctions appeared as dots, which were digitally measured and counted. Their average size was 0.40 mm in human and 0.49 mm in mice marrow. There were at least twice as many gap junctions in the femoral midshaft of 6-week-old mice (1.75 x 10(5)/mm3) as in those older than 12 weeks (0.89 x 10(5)/mm3). Most Cx43 was associated with collagen III+ endosteal and adventitial stromal cells and with megakaryocytes. Elsewhere, they were few and randomly distributed between all kinds of hematopoietic cells. In the femoral epiphysis of juvenile mice, stromal cell processes full of Cx43 enmeshed three to six layers of hematopoietic cells near the endosteum. The same pattern was seen in the midshaft of regenerating mouse marrow 3 to 5 days after cytotoxic treatment with 5-fluorouracil. Functional tests in cultures showed the transfer of small fluorescent dyes, Lucifer Yellow and 2',7'-bis-(2-carboxyethyl)-5, 6-carboxyfluorescein, between stromal cells and in rare cases between stromal and hematopoietic cells too. The stromal cells were densely packed with Cx43 and we found aggregates of connexon particles in their membrane replicas. In normocellular human bone marrow, gap junctions were as rare as in adult mouse and similarly distributed, except that they were also on adipocytic membranes. In a few leukemic samples, characterized by an increased stromal/hematopoietic cell ratio, there were two- to fourfold more Cx43 (2.8 x 10(5) to 3.9 x 10(5)/mm3) than in the normal (1.0 x 10(5) to 1.2 x 10(5)/mm3). The cases included a hypoplastic acute lymphoblastic leukemia, an acute myeloid leukemia (French-American-British classification M4-5), a case of myelodysplastic syndrome with elevated number of megakaryocytes, and a CD34+ acute hemoblastosis, probably acute myeloid leukemia (French-American-British classification M7). Taken together, our results indicate that direct cell-cell communication may be involved in hematopoiesis, ie, in developmentally active epiphyseal bone marrow and when there is a demand for progenitors in regeneration. However, gap junctions may not play as important a role in resting adult hematopoiesis and in leukemias.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alves L. A., Campos de Carvalho A. C., Cirne Lima E. O., Rocha e Souza C. M., Dardenne M., Spray D. C., Savino W. Functional gap junctions in thymic epithelial cells are formed by connexin 43. Eur J Immunol. 1995 Feb;25(2):431–437. doi: 10.1002/eji.1830250219. [DOI] [PubMed] [Google Scholar]
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Beresford J. N. Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop Relat Res. 1989 Mar;(240):270–280. [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. R., Hodgson G. S., Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- Breems D. A., Blokland E. A., Neben S., Ploemacher R. E. Frequency analysis of human primitive haematopoietic stem cell subsets using a cobblestone area forming cell assay. Leukemia. 1994 Jul;8(7):1095–1104. [PubMed] [Google Scholar]
- Bruzzone R., White T. W., Goodenough D. A. The cellular Internet: on-line with connexins. Bioessays. 1996 Sep;18(9):709–718. doi: 10.1002/bies.950180906. [DOI] [PubMed] [Google Scholar]
- Campbell F. R. Gap junctions between cells of bone marrow: an ultrastructural study using tannic acid. Anat Rec. 1980 Jan;196(1):101–107. doi: 10.1002/ar.1091960110. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Civitelli R., Beyer E. C., Warlow P. M., Robertson A. J., Geist S. T., Steinberg T. H. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest. 1993 May;91(5):1888–1896. doi: 10.1172/JCI116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K., Green L., Godwin A., Fletcher W. H. Connexin-43-type gap junctions mediate communication between bone marrow stromal cells. Blood. 1993 Jul 1;82(1):38–45. [PubMed] [Google Scholar]
- Giaume C., McCarthy K. D. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996 Aug;19(8):319–325. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y., Riley G. P., Watt S. M., Greaves M. F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. 1987 Mar 26-Apr 1Nature. 326(6111):403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- Green C. R., Peters N. S., Gourdie R. G., Rothery S., Severs N. J. Validation of immunohistochemical quantification in confocal scanning laser microscopy: a comparative assessment of gap junction size with confocal and ultrastructural techniques. J Histochem Cytochem. 1993 Sep;41(9):1339–1349. doi: 10.1177/41.9.8354875. [DOI] [PubMed] [Google Scholar]
- Green C. R., Severs N. J. Robert Feulgen Prize Lecture. Distribution and role of gap junctions in normal myocardium and human ischaemic heart disease. Histochemistry. 1993 Feb;99(2):105–120. doi: 10.1007/BF00571871. [DOI] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R., Radley J. M. The organization of hemopoietic tissue as inferred from the effects of 5-fluorouracil. Exp Hematol. 1982 Jan;10(1):26–35. [PubMed] [Google Scholar]
- Hotz-Wagenblatt A., Shalloway D. Gap junctional communication and neoplastic transformation. Crit Rev Oncog. 1993;4(5):541–558. [PubMed] [Google Scholar]
- Jones S. J., Gray C., Sakamaki H., Arora M., Boyde A., Gourdie R., Green C. The incidence and size of gap junctions between the bone cells in rat calvaria. Anat Embryol (Berl) 1993 Apr;187(4):343–352. doi: 10.1007/BF00185892. [DOI] [PubMed] [Google Scholar]
- Kittler E. L., McGrath H., Temeles D., Crittenden R. B., Kister V. K., Quesenberry P. J. Biologic significance of constitutive and subliminal growth factor production by bone marrow stroma. Blood. 1992 Jun 15;79(12):3168–3178. [PubMed] [Google Scholar]
- Krenacs T., van Dartel M., Lindhout E., Rosendaal M. Direct cell/cell communication in the lymphoid germinal center: connexin43 gap junctions functionally couple follicular dendritic cells to each other and to B lymphocytes. Eur J Immunol. 1997 Jun;27(6):1489–1497. doi: 10.1002/eji.1830270627. [DOI] [PubMed] [Google Scholar]
- Krenács T., Rosendaal M. Immunohistological detection of gap junctions in human lymphoid tissue: connexin43 in follicular dendritic and lymphoendothelial cells. J Histochem Cytochem. 1995 Nov;43(11):1125–1137. doi: 10.1177/43.11.7560895. [DOI] [PubMed] [Google Scholar]
- Kumar N. M., Gilula N. B. The gap junction communication channel. Cell. 1996 Feb 9;84(3):381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Rose B. The cell-cell channel in the control of growth. Semin Cell Biol. 1992 Feb;3(1):59–79. doi: 10.1016/s1043-4682(10)80008-x. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. The cell-to-cell channel of gap junctions. Cell. 1987 Mar 13;48(5):725–726. doi: 10.1016/0092-8674(87)90067-5. [DOI] [PubMed] [Google Scholar]
- Long M. W. Blood cell cytoadhesion molecules. Exp Hematol. 1992 Mar;20(3):288–301. [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Miller S. M., Garfield R. E., Daniel E. E. Improved propagation in myometrium associated with gap junctions during parturition. Am J Physiol. 1989 Jan;256(1 Pt 1):C130–C141. doi: 10.1152/ajpcell.1989.256.1.C130. [DOI] [PubMed] [Google Scholar]
- Rosendaal M. Gap junctions in blood forming tissues. Microsc Res Tech. 1995 Aug 1;31(5):396–407. doi: 10.1002/jemt.1070310509. [DOI] [PubMed] [Google Scholar]
- Rosendaal M., Green C. R., Rahman A., Morgan D. Up-regulation of the connexin43+ gap junction network in haemopoietic tissue before the growth of stem cells. J Cell Sci. 1994 Jan;107(Pt 1):29–37. doi: 10.1242/jcs.107.1.29. [DOI] [PubMed] [Google Scholar]
- Rosendaal M., Gregan A., Green C. R. Direct cell-cell communication in the blood-forming system. Tissue Cell. 1991;23(4):457–470. doi: 10.1016/0040-8166(91)90004-d. [DOI] [PubMed] [Google Scholar]
- Rosendaal M., Mayen A., de Koning A., Dunina-Barkovskaya T., Krenács T., Ploemacher R. Does transmembrane communication through gap junctions enable stem cells to overcome stromal inhibition? Leukemia. 1997 Aug;11(8):1281–1289. doi: 10.1038/sj.leu.2400744. [DOI] [PubMed] [Google Scholar]
- Severs N. J. The cardiac gap junction and intercalated disc. Int J Cardiol. 1990 Feb;26(2):137–173. doi: 10.1016/0167-5273(90)90030-9. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Tavassoli M., Crosby W. H. Bone marrow histogenesis: a comparison of fatty and red marrow. Science. 1970 Jul 17;169(3942):291–293. doi: 10.1126/science.169.3942.291. [DOI] [PubMed] [Google Scholar]
- Umezawa A., Hata J. Expression of gap-junctional protein (connexin 43 or alpha 1 gap junction) is down-regulated at the transcriptional level during adipocyte differentiation of H-1/A marrow stromal cells. Cell Struct Funct. 1992 Jun;17(3):177–184. doi: 10.1247/csf.17.177. [DOI] [PubMed] [Google Scholar]
- Warner A. Gap junctions in development--a perspective. Semin Cell Biol. 1992 Feb;3(1):81–91. doi: 10.1016/s1043-4682(10)80009-1. [DOI] [PubMed] [Google Scholar]
- Watanabe Y. Fine structure of bone marrow stroma. Nihon Ketsueki Gakkai Zasshi. 1985 Dec;48(8):1688–1700. [PubMed] [Google Scholar]