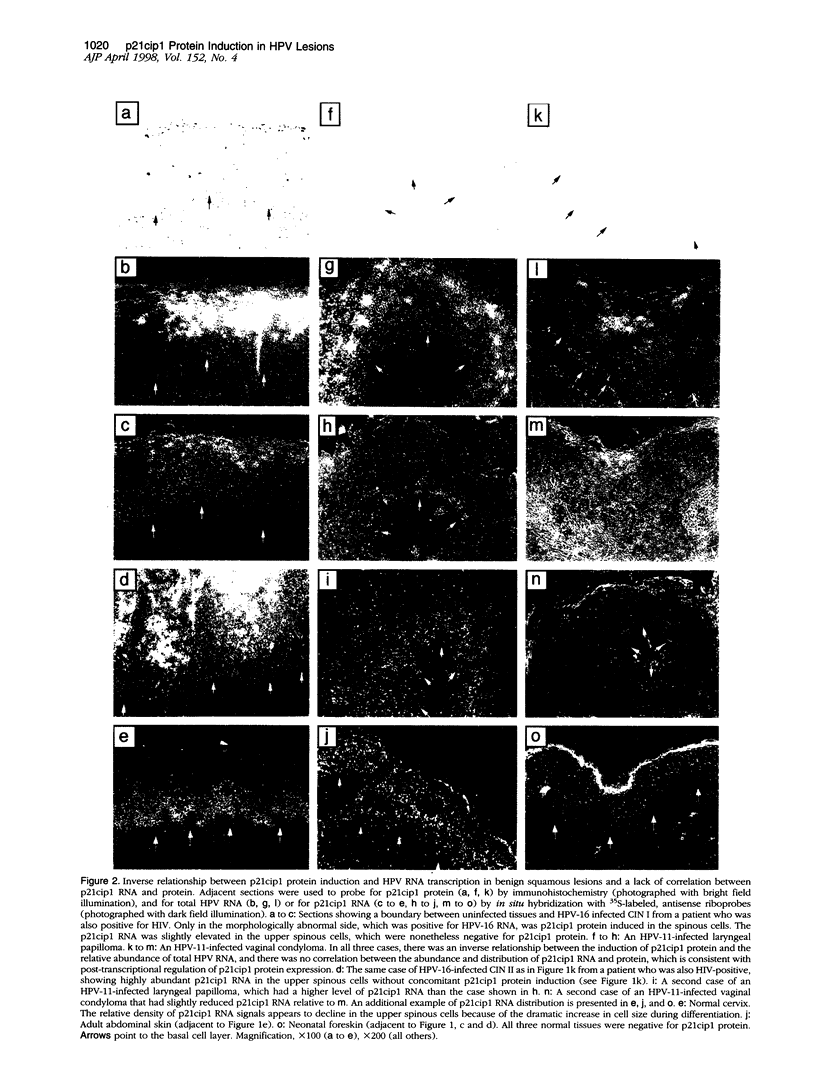
Abstract
Infections of the genital and oral epithelia by human papillomaviruses cause condylomata, papillomas, and squamous intraepithelial neoplasms, some of which can progress to invasive cancers. We describe an induction of p21cip1/WAF1/sdi1 protein in a fraction of the spinous cells in benign lesions and in cervical intraepithelial neoplasia grades I and II. The induction appears to be post-transcriptional and independent of p53. p21cip1 antigen-positive cells were sporadic in cervical intraepithelial neoplasia III and rare and focal in carcinomas. In contrast, p21cip1 protein was below or at the threshold of detection in the differentiated cells of normal squamous epithelia from different body sites despite an up-regulation of p21cip1 RNA. In cervical intraepithelial neoplasias from patients who were also positive for the human immunodeficiency virus, there was an additional increase in p21cip1 RNA in the upper spinous cells without concomitant p21cip1 protein induction. A consistent inverse relationship was observed between the p21cip1 protein induction and abundant human papillomavirus DNA and RNAs. We propose that p21cip1 protein induction is a novel host response that inhibits viral DNA replication and thus prevents elevated viral transcription. This hypothesis can partly account for the heterogeneity and the differentiation-dependent viral activities commonly observed in benign human papillomavirus lesions.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasofu M., Oda Y. Immunohistochemical detection of p53 in cervical epithelial lesions with or without infection of human papillomavirus types 16 and 18. Virchows Arch. 1995;425(6):593–602. doi: 10.1007/BF00199349. [DOI] [PubMed] [Google Scholar]
- Beyer-Finkler E., Stoler M. H., Girardi F., Pfister H. Cell differentiation-related gene expression of human papillomavirus 33. Med Microbiol Immunol. 1990;179(4):185–192. doi: 10.1007/BF00195249. [DOI] [PubMed] [Google Scholar]
- Busby-Earle R. M., Steel C. M., Williams A. R., Cohen B., Bird C. C. p53 mutations in cervical carcinogenesis--low frequency and lack of correlation with human papillomavirus status. Br J Cancer. 1994 Apr;69(4):732–737. doi: 10.1038/bjc.1994.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Schmidt-Grimminger D. C., Murant T., Broker T. R., Chow L. T. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995 Oct 1;9(19):2335–2349. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- Cox L. S., Lane D. P. Tumour suppressors, kinases and clamps: how p53 regulates the cell cycle in response to DNA damage. Bioessays. 1995 Jun;17(6):501–508. doi: 10.1002/bies.950170606. [DOI] [PubMed] [Google Scholar]
- Crook T., Vousden K. H. Properties of p53 mutations detected in primary and secondary cervical cancers suggest mechanisms of metastasis and involvement of environmental carcinogens. EMBO J. 1992 Nov;11(11):3935–3940. doi: 10.1002/j.1460-2075.1992.tb05487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum C. P., Nuovo G., Friedman D., Silverstein S. J. Accumulation of RNA homologous to human papillomavirus type 16 open reading frames in genital precancers. J Virol. 1988 Jan;62(1):84–90. doi: 10.1128/jvi.62.1.84-90.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto M. B., Yu Y., Wang X. F. Functional analysis of the transforming growth factor beta responsive elements in the WAF1/Cip1/p21 promoter. J Biol Chem. 1995 Dec 1;270(48):28623–28628. doi: 10.1074/jbc.270.48.28623. [DOI] [PubMed] [Google Scholar]
- Demeter L. M., Stoler M. H., Broker T. R., Chow L. T. Induction of proliferating cell nuclear antigen in differentiated keratinocytes of human papillomavirus-infected lesions. Hum Pathol. 1994 Apr;25(4):343–348. doi: 10.1016/0046-8177(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Dollard S. C., Wilson J. L., Demeter L. M., Bonnez W., Reichman R. C., Broker T. R., Chow L. T. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. OFF. Genes Dev. 1992 Jul;6(7):1131–1142. doi: 10.1101/gad.6.7.1131. [DOI] [PubMed] [Google Scholar]
- Dürst M., Glitz D., Schneider A., zur Hausen H. Human papillomavirus type 16 (HPV 16) gene expression and DNA replication in cervical neoplasia: analysis by in situ hybridization. Virology. 1992 Jul;189(1):132–140. doi: 10.1016/0042-6822(92)90688-l. [DOI] [PubMed] [Google Scholar]
- Elbendary A., Berchuck A., Davis P., Havrilesky L., Bast R. C., Jr, Iglehart J. D., Marks J. R. Transforming growth factor beta 1 can induce CIP1/WAF1 expression independent of the p53 pathway in ovarian cancer cells. Cell Growth Differ. 1994 Dec;5(12):1301–1307. [PubMed] [Google Scholar]
- Flores-Rozas H., Kelman Z., Dean F. B., Pan Z. Q., Harper J. W., Elledge S. J., O'Donnell M., Hurwitz J. Cdk-interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase delta holoenzyme. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8655–8659. doi: 10.1073/pnas.91.18.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouret P., Dabit D., Sibony M., Alili D., Commo F., Saint-Guily J. L., Callard P. Expression of p53 protein related to the presence of human papillomavirus infection in precancer lesions of the larynx. Am J Pathol. 1995 Mar;146(3):599–604. [PMC free article] [PubMed] [Google Scholar]
- Fredersdorf S., Milne A. W., Hall P. A., Lu X. Characterization of a panel of novel anti-p21Waf1/Cip1 monoclonal antibodies and immunochemical analysis of p21Waf1/Cip1 expression in normal human tissues. Am J Pathol. 1996 Mar;148(3):825–835. [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation. Curr Opin Cell Biol. 1990 Dec;2(6):1028–1035. doi: 10.1016/0955-0674(90)90152-5. [DOI] [PubMed] [Google Scholar]
- Funk J. O., Waga S., Harry J. B., Espling E., Stillman B., Galloway D. A. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997 Aug 15;11(16):2090–2100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis V., Rassidakis G., Karameris A., Giatromanolaki A., Barbatis C., Kittas C. Expression of p53 protein in laryngeal squamous cell carcinoma and dysplasia: possible correlation with human papillomavirus infection and clinicopathological findings. Virchows Arch. 1994;425(5):481–489. doi: 10.1007/BF00197551. [DOI] [PubMed] [Google Scholar]
- Halevy O., Novitch B. G., Spicer D. B., Skapek S. X., Rhee J., Hannon G. J., Beach D., Lassar A. B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995 Feb 17;267(5200):1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Healy E., Reynolds N. J., Smith M. D., Harrison D., Doherty E., Campbell C., Rees J. L. Up-regulation of p21WAF1/CIP1 in psoriasis and after the application of irritants and tape stripping. J Invest Dermatol. 1995 Aug;105(2):274–279. doi: 10.1111/1523-1747.ep12318430. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Rice K., Greenblatt M. S., Soussi T., Fuchs R., Sørlie T., Hovig E., Smith-Sørensen B., Montesano R., Harris C. C. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994 Sep;22(17):3551–3555. [PMC free article] [PubMed] [Google Scholar]
- Howes K. A., Ransom N., Papermaster D. S., Lasudry J. G., Albert D. M., Windle J. J. Apoptosis or retinoblastoma: alternative fates of photoreceptors expressing the HPV-16 E7 gene in the presence or absence of p53. Genes Dev. 1994 Jun 1;8(11):1300–1310. doi: 10.1101/gad.8.11.1300. [DOI] [PubMed] [Google Scholar]
- Inohara S., Kitagawa K., Kitano Y. Coexpression of p21Waf1/Cip1 and p53 in sun-exposed normal epidermis, but not in neoplastic epidermis. Br J Dermatol. 1996 Nov;135(5):717–720. [PubMed] [Google Scholar]
- Jiang H., Lin J., Su Z. Z., Collart F. R., Huberman E., Fisher P. B. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21, WAF1/CIP1, expression in the absence of p53. Oncogene. 1994 Nov;9(11):3397–3406. [PubMed] [Google Scholar]
- Jones D. L., Alani R. M., Münger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997 Aug 15;11(16):2101–2111. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Canman C. E., Leonard C. J. P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 1995 Mar;14(1):3–15. doi: 10.1007/BF00690207. [DOI] [PubMed] [Google Scholar]
- Kowalik T. F., DeGregori J., Schwarz J. K., Nevins J. R. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J Virol. 1995 Apr;69(4):2491–2500. doi: 10.1128/jvi.69.4.2491-2500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. R., Liu J. S., Broker T. R., Chow L. T. Cell-free replication of the human papillomavirus DNA with homologous viral E1 and E2 proteins and human cell extracts. J Biol Chem. 1994 Sep 30;269(39):24058–24065. [PubMed] [Google Scholar]
- La Thangue N. B. E2F and the molecular mechanisms of early cell-cycle control. Biochem Soc Trans. 1996 Feb;24(1):54–59. doi: 10.1042/bst0240054. [DOI] [PubMed] [Google Scholar]
- Liebermann D. A., Hoffman B., Steinman R. A. Molecular controls of growth arrest and apoptosis: p53-dependent and independent pathways. Oncogene. 1995 Jul 6;11(1):199–210. [PubMed] [Google Scholar]
- Liu M., Lee M. H., Cohen M., Bommakanti M., Freedman L. P. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996 Jan 15;10(2):142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- Macleod K. F., Sherry N., Hannon G., Beach D., Tokino T., Kinzler K., Vogelstein B., Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995 Apr 15;9(8):935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- Matsuta M., Kon S., Sasaki K., Matsuta M. Immunohistochemical detection of p21WAF1/CIP1 and p53 proteins in formalin-fixed paraffin-embedded tissue sections of squamous cell carcinoma of the skin. J Dermatol Sci. 1997 Mar;14(3):233–239. doi: 10.1016/s0923-1811(96)00579-8. [DOI] [PubMed] [Google Scholar]
- Meyers C., Frattini M. G., Hudson J. B., Laimins L. A. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992 Aug 14;257(5072):971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- Michieli P., Chedid M., Lin D., Pierce J. H., Mercer W. E., Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994 Jul 1;54(13):3391–3395. [PubMed] [Google Scholar]
- Missero C., Calautti E., Eckner R., Chin J., Tsai L. H., Livingston D. M., Dotto G. P. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C., Calautti E., Eckner R., Chin J., Tsai L. H., Livingston D. M., Dotto G. P. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K., Miyamoto S., Kato H., Imamura T., Nishida M., Yoshikawa Y., Nagata Y., Wake N. The role of p53 inactivation in human cervical cell carcinoma development. Br J Cancer. 1995 Feb;71(2):219–226. doi: 10.1038/bjc.1995.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. L., Wang T. S. Down-regulation of genes encoding DNA replication proteins during cell cycle exit. Cell Growth Differ. 1994 May;5(5):485–494. [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Palazzo J. P., Mercer W. E., Kovatich A. J., McHugh M. Immunohistochemical localization of p21(WAF1/CIP1) in normal, hyperplastic, and neoplastic uterine tissues. Hum Pathol. 1997 Jan;28(1):60–66. doi: 10.1016/s0046-8177(97)90280-x. [DOI] [PubMed] [Google Scholar]
- Pan H., Griep A. E. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994 Jun 1;8(11):1285–1299. doi: 10.1101/gad.8.11.1285. [DOI] [PubMed] [Google Scholar]
- Parker J. N., Zhao W., Askins K. J., Broker T. R., Chow L. T. Mutational analyses of differentiation-dependent human papillomavirus type 18 enhancer elements in epithelial raft cultures of neonatal foreskin keratinocytes. Cell Growth Differ. 1997 Jul;8(7):751–762. [PubMed] [Google Scholar]
- Parker S. B., Eichele G., Zhang P., Rawls A., Sands A. T., Bradley A., Olson E. N., Harper J. W., Elledge S. J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995 Feb 17;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- Pontén F., Berne B., Ren Z. P., Nistér M., Pontén J. Ultraviolet light induces expression of p53 and p21 in human skin: effect of sunscreen and constitutive p21 expression in skin appendages. J Invest Dermatol. 1995 Sep;105(3):402–406. doi: 10.1111/1523-1747.ep12321071. [DOI] [PubMed] [Google Scholar]
- Ruesch M. N., Laimins L. A. Initiation of DNA synthesis by human papillomavirus E7 oncoproteins is resistant to p21-mediated inhibition of cyclin E-cdk2 activity. J Virol. 1997 Jul;71(7):5570–5578. doi: 10.1128/jvi.71.7.5570-5578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Romanczuk H., Münger K., Huibregtse J. M., Mietz J. A., Howley P. M. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol. 1994;186:83–99. doi: 10.1007/978-3-642-78487-3_5. [DOI] [PubMed] [Google Scholar]
- Schiffman M. H. Epidemiology of cervical human papillomavirus infections. Curr Top Microbiol Immunol. 1994;186:55–81. doi: 10.1007/978-3-642-78487-3_4. [DOI] [PubMed] [Google Scholar]
- Schwaller J., Koeffler H. P., Niklaus G., Loetscher P., Nagel S., Fey M. F., Tobler A. Posttranscriptional stabilization underlies p53-independent induction of p21WAF1/CIP1/SDI1 in differentiating human leukemic cells. J Clin Invest. 1995 Mar;95(3):973–979. doi: 10.1172/JCI117806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995 May 15;9(10):1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Fornace A. J., Jr Genomic instability and the role of p53 mutations in cancer cells. Curr Opin Oncol. 1995 Jan;7(1):69–75. [PubMed] [Google Scholar]
- Steinman R. A., Hoffman B., Iro A., Guillouf C., Liebermann D. A., el-Houseini M. E. Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene. 1994 Nov;9(11):3389–3396. [PubMed] [Google Scholar]
- Stoler M. H., Broker T. R. In situ hybridization detection of human papillomavirus DNAs and messenger RNAs in genital condylomas and a cervical carcinoma. Hum Pathol. 1986 Dec;17(12):1250–1258. doi: 10.1016/s0046-8177(86)80569-x. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Rhodes C. R., Whitbeck A., Wolinsky S. M., Chow L. T., Broker T. R. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992 Feb;23(2):117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Wolinsky S. M., Whitbeck A., Broker T. R., Chow L. T. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology. 1989 Sep;172(1):331–340. doi: 10.1016/0042-6822(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Syrjänen S., Syrjänen K. An improved in situ DNA hybridization protocol for detection of human papillomavirus (HPV) DNA sequences in paraffin-embedded biopsies. J Virol Methods. 1986 Nov;14(3-4):293–304. doi: 10.1016/0166-0934(86)90031-5. [DOI] [PubMed] [Google Scholar]
- Tervahauta A. I., Syrjänen S. M., Mäntyjärvi R., Syrjänen K. J. Detection of p53 protein and Ki-67 proliferation antigen in human papillomavirus (HPV)-positive and HPV-negative cervical lesions by immunohistochemical double-staining. Cytopathology. 1994 Oct;5(5):282–293. doi: 10.1111/j.1365-2303.1994.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Tommasino M., Crawford L. Human papillomavirus E6 and E7: proteins which deregulate the cell cycle. Bioessays. 1995 Jun;17(6):509–518. doi: 10.1002/bies.950170607. [DOI] [PubMed] [Google Scholar]
- Tron V. A., Tang L., Yong W. P., Trotter M. J. Differentiation-associated overexpression of the cyclin-dependent kinase inhibitor p21waf-1 in human cutaneous squamous cell carcinoma. Am J Pathol. 1996 Oct;149(4):1139–1146. [PMC free article] [PubMed] [Google Scholar]
- Waga S., Hannon G. J., Beach D., Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994 Jun 16;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Werness B. A., Wang H. Q., Chance J., Goldstein D. J. p53-independent expression of p21waf1/cip1 in preinvasive and invasive squamous neoplasms of the uterine cervix. Mod Pathol. 1997 Jun;10(6):578–584. [PubMed] [Google Scholar]
- Wilbur D. C., Reichman R. C., Stoler M. H. Detection of infection by human papillomavirus in genital condylomata. A comparison study using immunocytochemistry and in situ nucleic acid hybridization. Am J Clin Pathol. 1988 Apr;89(4):505–510. doi: 10.1093/ajcp/89.4.505. [DOI] [PubMed] [Google Scholar]
- Wu X., Levine A. J. p53 and E2F-1 cooperate to mediate apoptosis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Kuppuswamy D., Li Y., Livanos E. M., Hixon M., White A., Beach D., Tlsty T. D. Alteration of cell cycle kinase complexes in human papillomavirus E6- and E7-expressing fibroblasts precedes neoplastic transformation. J Virol. 1996 Feb;70(2):999–1008. doi: 10.1128/jvi.70.2.999-1008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y. X., el-Deiry W. S. Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene. 1996 Apr 4;12(7):1557–1564. [PubMed] [Google Scholar]
- Zhang W., Grasso L., McClain C. D., Gambel A. M., Cha Y., Travali S., Deisseroth A. B., Mercer W. E. p53-independent induction of WAF1/CIP1 in human leukemia cells is correlated with growth arrest accompanying monocyte/macrophage differentiation. Cancer Res. 1995 Feb 1;55(3):668–674. [PubMed] [Google Scholar]
- Zhao W., Chow L. T., Broker T. R. Transcription activities of human papillomavirus type 11 E6 promoter-proximal elements in raft and submerged cultures of foreskin keratinocytes. J Virol. 1997 Nov;71(11):8832–8840. doi: 10.1128/jvi.71.11.8832-8840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Waldman T., Oliner J. D., Velculescu V. E., Burrell M., Hill D. E., Healy E., Rees J. L., Hamilton S. R. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995 Jul 1;55(13):2910–2919. [PubMed] [Google Scholar]
- zur Hausen H., de Villiers E. M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]