Abstract
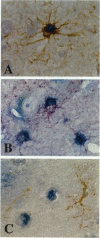
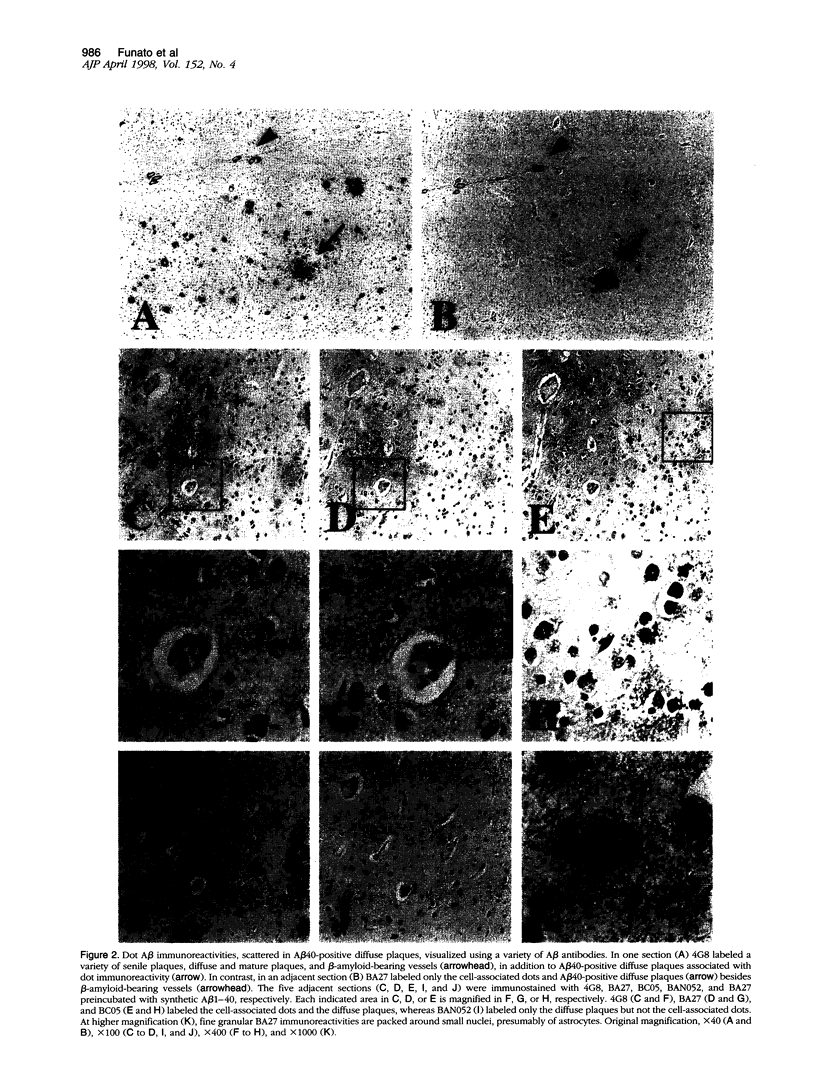
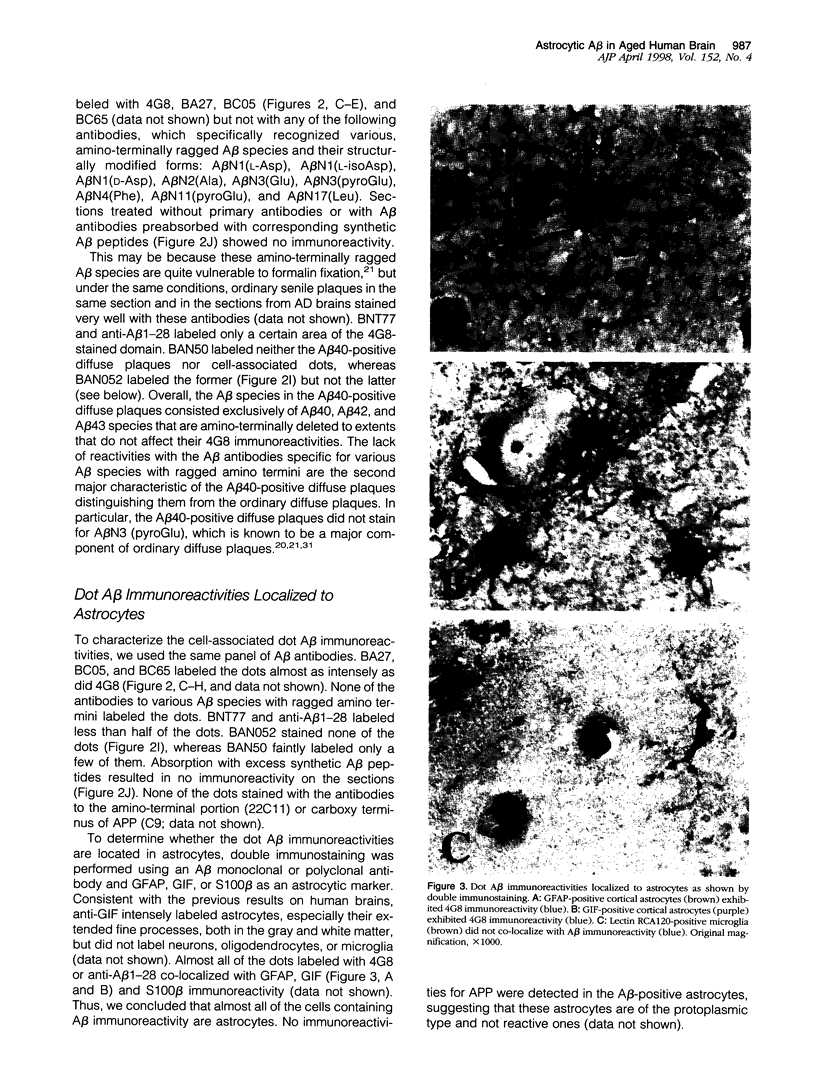
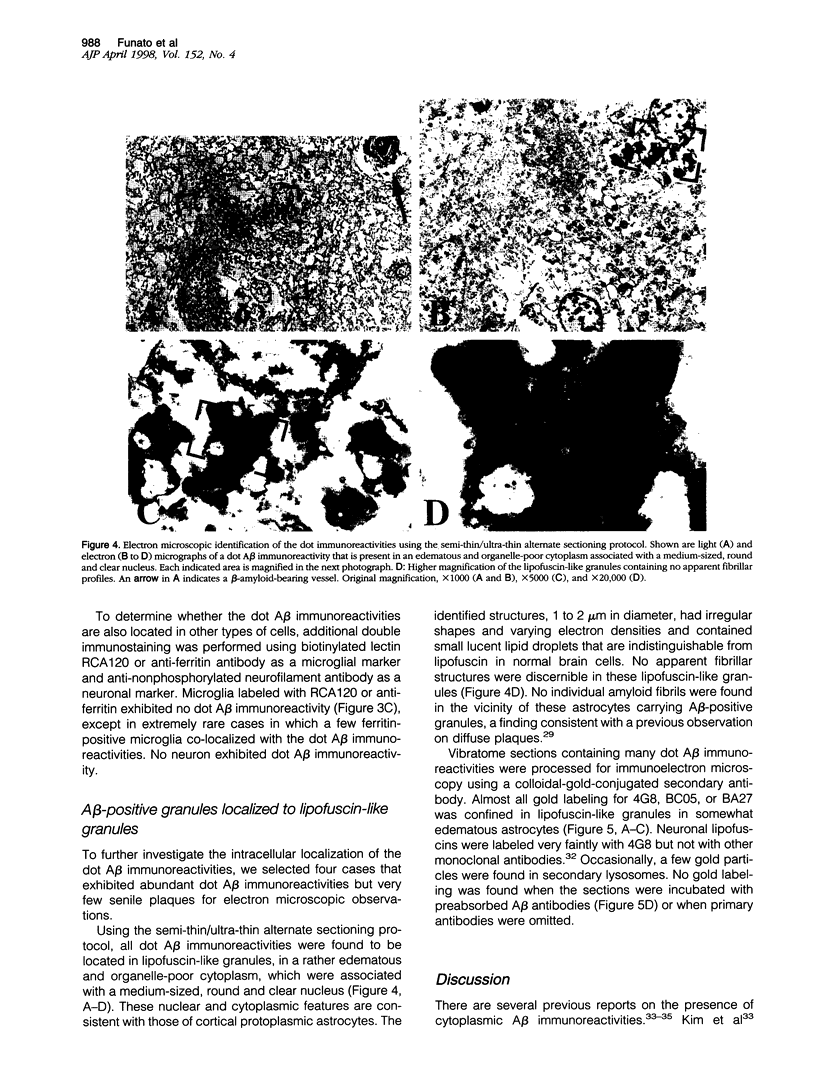
Amyloid beta-protein (Abeta) is the major component of senile plaques that emerge in the cortex during aging and appear most abundantly in Alzheimer's disease. In the course of our immunocytochemical study on a large number of autopsy cases, we noticed, in many aged nondemented cases, the presence of unique diffuse plaques in the cortex distinct from ordinary diffuse plaques by immunocytochemistry. The former were amorphous, very faintly Abeta-immunoreactive plaques resembling diffuse plaques, but they stained for Abeta40 and were associated with small cells containing Abeta-positive granules. A panel of amino- and carboxyl-terminal-specific Abeta antibodies showed that such Abeta40-positive diffuse plaques and cell-associated granules were composed exclusively of amino-terminally deleted Abeta terminating at Abeta40, -42, and -43. Double immunostaining also showed that those Abeta-immunoreactive granules are located in astrocytes and not in microglia or neurons. Immunoelectron microscopy revealed that nonfibrillar Abeta immunoreactivity was located within lipofuscin-like granules in somewhat swollen astrocytes. These findings raise the possibility that astrocytes take up Abeta and attempt to degrade it in lysosomes in the aged brain.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama H., Kondo H., Mori H., Kametani F., Nishimura T., Ikeda K., Kato M., McGeer P. L. The amino-terminally truncated forms of amyloid beta-protein in brain macrophages in the ischemic lesions of Alzheimer's disease patients. Neurosci Lett. 1996 Nov 22;219(2):115–118. doi: 10.1016/s0304-3940(96)13197-9. [DOI] [PubMed] [Google Scholar]
- Akiyama H., Schwab C., Kondo H., Mori H., Kametani F., Ikeda K., McGeer P. L. Granules in glial cells of patients with Alzheimer's disease are immunopositive for C-terminal sequences of beta-amyloid protein. Neurosci Lett. 1996 Mar 15;206(2-3):169–172. doi: 10.1016/s0304-3940(96)12474-5. [DOI] [PubMed] [Google Scholar]
- Asami-Odaka A., Ishibashi Y., Kikuchi T., Kitada C., Suzuki N. Long amyloid beta-protein secreted from wild-type human neuroblastoma IMR-32 cells. Biochemistry. 1995 Aug 15;34(32):10272–10278. doi: 10.1021/bi00032a022. [DOI] [PubMed] [Google Scholar]
- Bancher C., Grundke-Iqbal I., Iqbal K., Kim K. S., Wisniewski H. M. Immunoreactivity of neuronal lipofuscin with monoclonal antibodies to the amyloid beta-protein. Neurobiol Aging. 1989 Mar-Apr;10(2):125–132. doi: 10.1016/0197-4580(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Boland K., Behrens M., Choi D., Manias K., Perlmutter D. H. The serpin-enzyme complex receptor recognizes soluble, nontoxic amyloid-beta peptide but not aggregated, cytotoxic amyloid-beta peptide. J Biol Chem. 1996 Jul 26;271(30):18032–18044. doi: 10.1074/jbc.271.30.18032. [DOI] [PubMed] [Google Scholar]
- Cataldo A. M., Nixon R. A. Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc Natl Acad Sci U S A. 1990 May;87(10):3861–3865. doi: 10.1073/pnas.87.10.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G., Pileri S., Parravicini C., Becker M. H., Poggi S., Bifulco C., Key G., D'Amato L., Sabattini E., Feudale E. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993 Oct;171(2):83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- Dickson D. W. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997 Apr;56(4):321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- El Khoury J., Hickman S. E., Thomas C. A., Cao L., Silverstein S. C., Loike J. D. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996 Aug 22;382(6593):716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Evin G., Cappai R., Li Q. X., Culvenor J. G., Small D. H., Beyreuther K., Masters C. L. Candidate gamma-secretases in the generation of the carboxyl terminus of the Alzheimer's disease beta A4 amyloid: possible involvement of cathepsin D. Biochemistry. 1995 Oct 31;34(43):14185–14192. doi: 10.1021/bi00043a024. [DOI] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Younkin L. H., Selkoe D. J., Younkin S. G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992 Feb 7;255(5045):728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Fleming J., Tung Y. C., Lassmann H., Iqbal K., Joshi J. G. Ferritin is a component of the neuritic (senile) plaque in Alzheimer dementia. Acta Neuropathol. 1990;81(2):105–110. doi: 10.1007/BF00334497. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., George L., Tung Y. C., Kim K. S., Wisniewski H. M. Amyloid protein and neurofibrillary tangles coexist in the same neuron in Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2853–2857. doi: 10.1073/pnas.86.8.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Koo E. H., Mellon A., Hung A. Y., Selkoe D. J. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992 Jun 11;357(6378):500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- Hozumi I., Inuzuka T., Hiraiwa M., Uchida Y., Anezaki T., Ishiguro H., Kobayashi H., Uda Y., Miyatake T., Tsuji S. Changes of growth inhibitory factor after stab wounds in rat brain. Brain Res. 1995 Aug 7;688(1-2):143–148. doi: 10.1016/0006-8993(95)00522-r. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Mann D. M., Odaka A., Suzuki N., Ihara Y. Amyloid beta protein (A beta) deposition: A beta 42(43) precedes A beta 40 in Down syndrome. Ann Neurol. 1995 Mar;37(3):294–299. doi: 10.1002/ana.410370305. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron. 1994 Jul;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T., Saido T. C., Mann D. M., Lee V. M., Trojanowski J. Q. Full-length amyloid-beta (1-42(43)) and amino-terminally modified and truncated amyloid-beta 42(43) deposit in diffuse plaques. Am J Pathol. 1996 Dec;149(6):1823–1830. [PMC free article] [PubMed] [Google Scholar]
- Knauer M. F., Soreghan B., Burdick D., Kosmoski J., Glabe C. G. Intracellular accumulation and resistance to degradation of the Alzheimer amyloid A4/beta protein. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7437–7441. doi: 10.1073/pnas.89.16.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladror U. S., Snyder S. W., Wang G. T., Holzman T. F., Krafft G. A. Cleavage at the amino and carboxyl termini of Alzheimer's amyloid-beta by cathepsin D. J Biol Chem. 1994 Jul 15;269(28):18422–18428. [PubMed] [Google Scholar]
- Mannoji H., Yeger H., Becker L. E. A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol. 1986;71(3-4):341–343. doi: 10.1007/BF00688060. [DOI] [PubMed] [Google Scholar]
- Martin L. J., Pardo C. A., Cork L. C., Price D. L. Synaptic pathology and glial responses to neuronal injury precede the formation of senile plaques and amyloid deposits in the aging cerebral cortex. Am J Pathol. 1994 Dec;145(6):1358–1381. [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu A., Quon D., Cordell B. beta-Amyloid peptide produced in vitro is degraded by proteinases released by cultured cells. J Biol Chem. 1995 Jan 20;270(3):1369–1374. doi: 10.1074/jbc.270.3.1369. [DOI] [PubMed] [Google Scholar]
- Nordstedt C., Näslund J., Tjernberg L. O., Karlström A. R., Thyberg J., Terenius L. The Alzheimer A beta peptide develops protease resistance in association with its polymerization into fibrils. J Biol Chem. 1994 Dec 9;269(49):30773–30776. [PubMed] [Google Scholar]
- Otsuka N., Tomonaga M., Ikeda K. Rapid appearance of beta-amyloid precursor protein immunoreactivity in damaged axons and reactive glial cells in rat brain following needle stab injury. Brain Res. 1991 Dec 24;568(1-2):335–338. doi: 10.1016/0006-8993(91)91422-w. [DOI] [PubMed] [Google Scholar]
- Paresce D. M., Ghosh R. N., Maxfield F. R. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron. 1996 Sep;17(3):553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Overman M. J., Cotman C. W. Amino-terminal deletions enhance aggregation of beta-amyloid peptides in vitro. J Biol Chem. 1995 Oct 13;270(41):23895–23898. doi: 10.1074/jbc.270.41.23895. [DOI] [PubMed] [Google Scholar]
- Qiu W. Q., Borth W., Ye Z., Haass C., Teplow D. B., Selkoe D. J. Degradation of amyloid beta-protein by a serine protease-alpha2-macroglobulin complex. J Biol Chem. 1996 Apr 5;271(14):8443–8451. doi: 10.1074/jbc.271.14.8443. [DOI] [PubMed] [Google Scholar]
- Saftig P., Peters C., von Figura K., Craessaerts K., Van Leuven F., De Strooper B. Amyloidogenic processing of human amyloid precursor protein in hippocampal neurons devoid of cathepsin D. J Biol Chem. 1996 Nov 1;271(44):27241–27244. doi: 10.1074/jbc.271.44.27241. [DOI] [PubMed] [Google Scholar]
- Saido T. C., Iwatsubo T., Mann D. M., Shimada H., Ihara Y., Kawashima S. Dominant and differential deposition of distinct beta-amyloid peptide species, A beta N3(pE), in senile plaques. Neuron. 1995 Feb;14(2):457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- Shaffer L. M., Dority M. D., Gupta-Bansal R., Frederickson R. C., Younkin S. G., Brunden K. R. Amyloid beta protein (A beta) removal by neuroglial cells in culture. Neurobiol Aging. 1995 Sep-Oct;16(5):737–745. doi: 10.1016/0197-4580(95)00055-j. [DOI] [PubMed] [Google Scholar]
- Shin R. W., Iwaki T., Kitamoto T., Tateishi J. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer's disease brain tissues. Lab Invest. 1991 May;64(5):693–702. [PubMed] [Google Scholar]
- Shinkai Y., Yoshimura M., Ito Y., Odaka A., Suzuki N., Yanagisawa K., Ihara Y. Amyloid beta-proteins 1-40 and 1-42(43) in the soluble fraction of extra- and intracranial blood vessels. Ann Neurol. 1995 Sep;38(3):421–428. doi: 10.1002/ana.410380312. [DOI] [PubMed] [Google Scholar]
- Stern R. A., Otvos L., Jr, Trojanowski J. Q., Lee V. M. Monoclonal antibodies to a synthetic peptide homologous with the first 28 amino acids of Alzheimer's disease beta-protein recognize amyloid and diverse glial and neuronal cell types in the central nervous system. Am J Pathol. 1989 May;134(5):973–978. [PMC free article] [PubMed] [Google Scholar]
- Stern R. A., Trojanowski J. Q., Lee V. M. Antibodies to the beta-amyloid peptide cross-react with conformational epitopes in human fibrinogen subunits from peripheral blood. FEBS Lett. 1990 May 7;264(1):43–47. doi: 10.1016/0014-5793(90)80760-g. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Utsuyama M., Kurashima C., Mori H., Hirokawa K. Monoclonal antibody to beta peptide, recognizing amyloid deposits, neuronal cells and lipofuscin pigments in systemic organs. Acta Neuropathol. 1993;85(2):159–166. doi: 10.1007/BF00227763. [DOI] [PubMed] [Google Scholar]
- Tamaoka A., Endoh R., Shoji S., Takahashi H., Hirokawa K., Teplow D. B., Selkoe D. J., Mori H. Antibodies to amyloid beta protein (A beta) crossreact with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Neurobiol Aging. 1996 May-Jun;17(3):405–414. doi: 10.1016/0197-4580(96)00031-0. [DOI] [PubMed] [Google Scholar]
- Tierney M. C., Fisher R. H., Lewis A. J., Zorzitto M. L., Snow W. G., Reid D. W., Nieuwstraten P. The NINCDS-ADRDA Work Group criteria for the clinical diagnosis of probable Alzheimer's disease: a clinicopathologic study of 57 cases. Neurology. 1988 Mar;38(3):359–364. doi: 10.1212/wnl.38.3.359. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Takio K., Titani K., Ihara Y., Tomonaga M. The growth inhibitory factor that is deficient in the Alzheimer's disease brain is a 68 amino acid metallothionein-like protein. Neuron. 1991 Aug;7(2):337–347. doi: 10.1016/0896-6273(91)90272-2. [DOI] [PubMed] [Google Scholar]
- Vinores S. A., Herman M. M. Phagocytosis of myelin by astrocytes in explants of adult rabbit cerebral white matter maintained on Gelfoam matrix. J Neuroimmunol. 1993 Mar;43(1-2):169–176. doi: 10.1016/0165-5728(93)90088-g. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Weigel J. Migration of perivascular cells into the neuropil and their involvement in beta-amyloid plaque formation. Acta Neuropathol. 1993;85(6):586–595. doi: 10.1007/BF00334667. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Morimatsu M., Hirai S., Takahashi K. Alzheimer's neurofibrillary tangles are penetrated by astroglial processes and appear eosinophilic in their final stages. Acta Neuropathol. 1987;72(3):214–217. doi: 10.1007/BF00691092. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Nakazato Y., Hirai S., Shoji M., Harigaya Y. Electron micrograph of diffuse plaques. Initial stage of senile plaque formation in the Alzheimer brain. Am J Pathol. 1989 Oct;135(4):593–597. [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T., Yamaguchi H., Nakazato Y., Ishiguro K., Kawarabayashi T., Hirai S. Ultrastructural characterization of cerebellar diffuse plaques in Alzheimer's disease. J Neuropathol Exp Neurol. 1992 May;51(3):281–286. doi: 10.1097/00005072-199205000-00006. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Chen X., Fu J., Chen M., Zhu H., Roher A., Slattery T., Zhao L., Nagashima M., Morser J. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996 Aug 22;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K., Odaka A., Suzuki N., Ihara Y. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer's disease. Nat Med. 1995 Oct;1(10):1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]