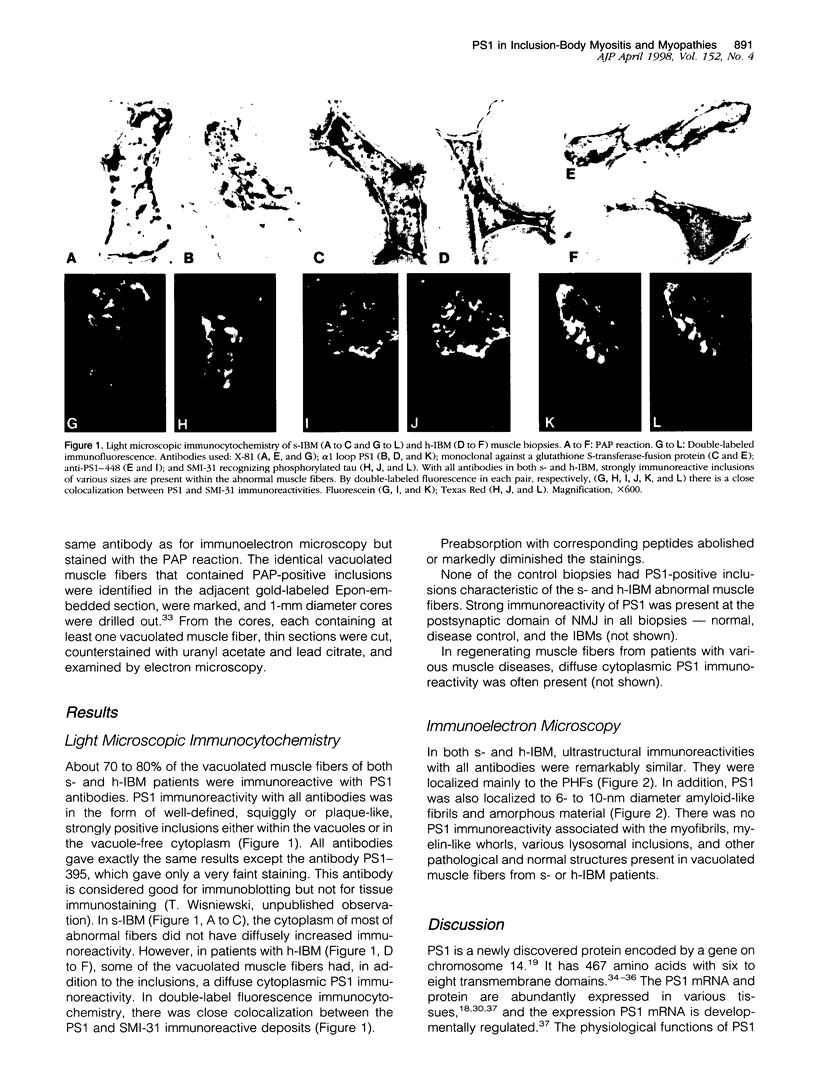
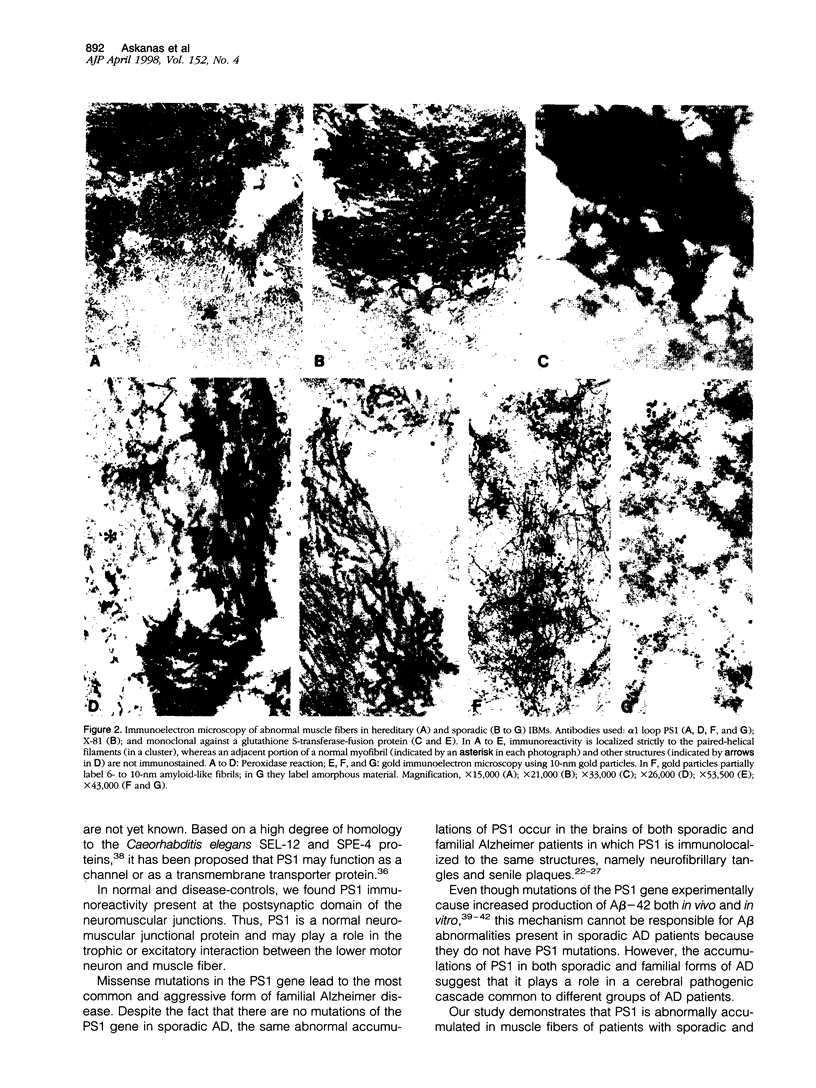
Abstract
Sporadic inclusion-body myositis (s-IBM) is the most common progressive muscle disease of older persons. The muscle biopsy demonstrates mononuclear cell inflammation and vacuolated muscle fibers containing paired helical filaments and 6- to 10-nm fibrils, both resembling those of Alzheimer disease brain and Congo red positivity. The term hereditary inclusion-body myopathies (h-IBMs) designates autosomal-recessive or autosomal-dominant disorders with muscle biopsies cytopathologically similar to s-IBM but without inflammation. Vacuolated muscle fibers of both s-IBM and the h-IBMs contain accumulations of several "Alzheimer-characteristic proteins" including beta-amyloid protein and beta-amyloid precursor protein, and their paired helical filaments are composed of phosphorylated tau. We used six well characterized antibodies against several residues of presenilin 1 (PS1) to immunostain muscle biopsies of 12 patients with s-IBM, 5 patients with autosomal-recessive inclusion-body myopathy, and 16 normal and disease controls. Seventy to eighty percent of the vacuolated muscle fibers of both s-IBM and autosomal-recessive inclusion-body myopathy had inclusions that were strongly PS1-immunoreactive, which by immunoelectron microscopy localized mainly to paired helical filaments and 6- to 10-nm filaments. None of the control biopsies had PS1-positive inclusions characteristic of the s- and h-IBM abnormal muscle fibers. Mutations of the newly discovered PS1 gene are responsible for early-onset familial Alzheimer disease (AD), and PS1 is abnormally accumulated in sporadic and familial AD brain. Our study provides the first demonstration of PS1 abnormality in non-neural tissue and in diseases other than AD and suggests that the cytopathogenesis in AD brain and IBM muscle may share similarities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askanas V., Alvarez R. B., Engel W. K. beta-Amyloid precursor epitopes in muscle fibers of inclusion body myositis. Ann Neurol. 1993 Oct;34(4):551–560. doi: 10.1002/ana.410340408. [DOI] [PubMed] [Google Scholar]
- Askanas V., Alvarez R. B., Mirabella M., Engel W. K. Use of anti-neurofilament antibody to identify paired-helical filaments in inclusion-body myositis. Ann Neurol. 1996 Mar;39(3):389–391. doi: 10.1002/ana.410390318. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Alvarez R. B. Enhanced detection of congo-red-positive amyloid deposits in muscle fibers of inclusion body myositis and brain of Alzheimer's disease using fluorescence technique. Neurology. 1993 Jun;43(6):1265–1267. doi: 10.1212/wnl.43.6.1265-a. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Alvarez R. B. Light and electron microscopic localization of beta-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol. 1992 Jul;141(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Bilak M., Alvarez R. B., Selkoe D. J. Twisted tubulofilaments of inclusion body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am J Pathol. 1994 Jan;144(1):177–187. [PMC free article] [PubMed] [Google Scholar]
- Askanas V., Engel W. K. New advances in inclusion-body myositis. Curr Opin Rheumatol. 1993 Nov;5(6):732–741. doi: 10.1097/00002281-199305060-00007. [DOI] [PubMed] [Google Scholar]
- Askanas V., McFerrin J., Alvarez R. B., Baqué S., Engel W. K. Beta APP gene transfer into cultured human muscle induces inclusion-body myositis aspects. Neuroreport. 1997 Jul 7;8(9-10):2155–2158. doi: 10.1097/00001756-199707070-00012. [DOI] [PubMed] [Google Scholar]
- Askanas V., McFerrin J., Baqué S., Alvarez R. B., Sarkozi E., Engel W. K. Transfer of beta-amyloid precursor protein gene using adenovirus vector causes mitochondrial abnormalities in cultured normal human muscle. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1314–1319. doi: 10.1073/pnas.93.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askanas V. New developments in hereditary inclusion body myopathies. Ann Neurol. 1997 Apr;41(4):421–422. doi: 10.1002/ana.410410403. [DOI] [PubMed] [Google Scholar]
- Askanas V., Serdaroglu P., Engel W. K., Alvarez R. B. Immunolocalization of ubiquitin in muscle biopsies of patients with inclusion body myositis and oculopharyngeal muscular dystrophy. Neurosci Lett. 1991 Sep 2;130(1):73–76. doi: 10.1016/0304-3940(91)90230-q. [DOI] [PubMed] [Google Scholar]
- Bilak M., Askanas V., Engel W. K. Strong immunoreactivity of alpha 1-antichymotrypsin co-localizes with beta-amyloid protein and ubiquitin in vacuolated muscle fibers of inclusion-body myositis. Acta Neuropathol. 1993;85(4):378–382. doi: 10.1007/BF00334447. [DOI] [PubMed] [Google Scholar]
- Borchelt D. R., Thinakaran G., Eckman C. B., Lee M. K., Davenport F., Ratovitsky T., Prada C. M., Kim G., Seekins S., Yager D. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996 Nov;17(5):1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Busciglio J., Hartmann H., Lorenzo A., Wong C., Baumann K., Sommer B., Staufenbiel M., Yankner B. A. Neuronal localization of presenilin-1 and association with amyloid plaques and neurofibrillary tangles in Alzheimer's disease. J Neurosci. 1997 Jul 1;17(13):5101–5107. doi: 10.1523/JNEUROSCI.17-13-05101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan A., Thinakaran G., Borchelt D. R., Slunt H. H., Ratovitsky T., Podlisny M., Selkoe D. J., Seeger M., Gandy S. E., Price D. L. Protein topology of presenilin 1. Neuron. 1996 Nov;17(5):1023–1030. doi: 10.1016/s0896-6273(00)80232-9. [DOI] [PubMed] [Google Scholar]
- Duff K., Eckman C., Zehr C., Yu X., Prada C. M., Perez-tur J., Hutton M., Buee L., Harigaya Y., Yager D. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996 Oct 24;383(6602):710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- ENGEL W. K., CUNNINGHAM G. G. RAPID EXAMINATION OF MUSCLE TISSUE. AN IMPROVED TRICHROME METHOD FOR FRESH-FROZEN BIOPSY SECTIONS. Neurology. 1963 Nov;13:919–923. doi: 10.1212/wnl.13.11.919. [DOI] [PubMed] [Google Scholar]
- Engel W. K. Muscle biopsies in neuromuscular diseases. Pediatr Clin North Am. 1967 Nov;14(4):963–995. doi: 10.1016/s0031-3955(16)32067-3. [DOI] [PubMed] [Google Scholar]
- Gorycki M. A., Askanas V. Improved technique for electron microscopy of cultured cells. Stain Technol. 1977 Sep;52(5):249–254. doi: 10.3109/10520297709116788. [DOI] [PubMed] [Google Scholar]
- Haass C. Presenilins: genes for life and death. Neuron. 1997 May;18(5):687–690. doi: 10.1016/s0896-6273(00)80309-8. [DOI] [PubMed] [Google Scholar]
- Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997 Apr;20(4):154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- Huynh D. P., Vinters H. V., Ho D. H., Ho V. V., Pulst S. M. Neuronal expression and intracellular localization of presenilins in normal and Alzheimer disease brains. J Neuropathol Exp Neurol. 1997 Sep;56(9):1009–1017. doi: 10.1097/00005072-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T., Asaka T., Saito M., Tanaka H., Higuchi S., Tanaka K., Saida K., Uyama E., Mizusawa H., Fukuhara N. Gene locus for autosomal recessive distal myopathy with rimmed vacuoles maps to chromosome 9. Ann Neurol. 1997 Apr;41(4):432–437. doi: 10.1002/ana.410410405. [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Froelich S., Lannfelt L., Cowburn R. F. Quantification of presenilin-1 mRNA in Alzheimer's disease brains. FEBS Lett. 1996 Oct 7;394(3):279–284. doi: 10.1016/0014-5793(96)00969-6. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Dickson D. W., Davies P., Yen S. H. Recognition of tau epitopes by anti-neurofilament antibodies that bind to Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1987 May;84(10):3410–3414. doi: 10.1073/pnas.84.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. K., Slunt H. H., Martin L. J., Thinakaran G., Kim G., Gandy S. E., Seeger M., Koo E., Price D. L., Sisodia S. S. Expression of presenilin 1 and 2 (PS1 and PS2) in human and murine tissues. J Neurosci. 1996 Dec 1;16(23):7513–7525. doi: 10.1523/JNEUROSCI.16-23-07513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S., Chiesa R., Harris D. A. Evidence for a six-transmembrane domain structure of presenilin 1. J Biol Chem. 1997 May 2;272(18):12047–12051. doi: 10.1074/jbc.272.18.12047. [DOI] [PubMed] [Google Scholar]
- Lemere C. A., Lopera F., Kosik K. S., Lendon C. L., Ossa J., Saido T. C., Yamaguchi H., Ruiz A., Martinez A., Madrigal L. The E280A presenilin 1 Alzheimer mutation produces increased A beta 42 deposition and severe cerebellar pathology. Nat Med. 1996 Oct;2(10):1146–1150. doi: 10.1038/nm1096-1146. [DOI] [PubMed] [Google Scholar]
- Levey A. I., Heilman C. J., Lah J. J., Nash N. R., Rees H. D., Wakai M., Mirra S. S., Rye D. B., Nochlin D., Bird T. D. Presenilin-1 protein expression in familial and sporadic Alzheimer's disease. Ann Neurol. 1997 Jun;41(6):742–753. doi: 10.1002/ana.410410610. [DOI] [PubMed] [Google Scholar]
- Levitan D., Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995 Sep 28;377(6547):351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Li X., Greenwald I. Membrane topology of the C. elegans SEL-12 presenilin. Neuron. 1996 Nov;17(5):1015–1021. doi: 10.1016/s0896-6273(00)80231-7. [DOI] [PubMed] [Google Scholar]
- Lippa C. F., Saunders A. M., Smith T. W., Swearer J. M., Drachman D. A., Ghetti B., Nee L., Pulaski-Salo D., Dickson D., Robitaille Y. Familial and sporadic Alzheimer's disease: neuropathology cannot exclude a final common pathway. Neurology. 1996 Feb;46(2):406–412. doi: 10.1212/wnl.46.2.406. [DOI] [PubMed] [Google Scholar]
- Mendell J. R., Sahenk Z., Gales T., Paul L. Amyloid filaments in inclusion body myositis. Novel findings provide insight into nature of filaments. Arch Neurol. 1991 Dec;48(12):1229–1234. doi: 10.1001/archneur.1991.00530240033013. [DOI] [PubMed] [Google Scholar]
- Mirabella M., Alvarez R. B., Bilak M., Engel W. K., Askanas V. Difference in expression of phosphorylated tau epitopes between sporadic inclusion-body myositis and hereditary inclusion-body myopathies. J Neuropathol Exp Neurol. 1996 Jul;55(7):774–786. doi: 10.1097/00005072-199607000-00003. [DOI] [PubMed] [Google Scholar]
- Mirabella M., Alvarez R. B., Engel W. K., Weisgraber K. H., Askanas V. Apolipoprotein E and apolipoprotein E messenger RNA in muscle of inclusion body myositis and myopathies. Ann Neurol. 1996 Dec;40(6):864–872. doi: 10.1002/ana.410400608. [DOI] [PubMed] [Google Scholar]
- Mitrani-Rosenbaum S., Argov Z., Blumenfeld A., Seidman C. E., Seidman J. G. Hereditary inclusion body myopathy maps to chromosome 9p1-q1. Hum Mol Genet. 1996 Jan;5(1):159–163. doi: 10.1093/hmg/5.1.159. [DOI] [PubMed] [Google Scholar]
- Murphy G. M., Jr, Forno L. S., Ellis W. G., Nochlin D., Levy-Lahad E., Poorkaj P., Bird T. D., Jiang Z., Cordell B. Antibodies to presenilin proteins detect neurofibrillary tangles in Alzheimer's disease. Am J Pathol. 1996 Dec;149(6):1839–1846. [PMC free article] [PubMed] [Google Scholar]
- Podlisny M. B., Citron M., Amarante P., Sherrington R., Xia W., Zhang J., Diehl T., Levesque G., Fraser P., Haass C. Presenilin proteins undergo heterogeneous endoproteolysis between Thr291 and Ala299 and occur as stable N- and C-terminal fragments in normal and Alzheimer brain tissue. Neurobiol Dis. 1997;3(4):325–337. doi: 10.1006/nbdi.1997.0129. [DOI] [PubMed] [Google Scholar]
- Sarkozi E., Askanas V., Johnson S. A., Engel W. K., Alvarez R. B. beta-Amyloid precursor protein mRNA is increased in inclusion-body myositis muscle. Neuroreport. 1993 Jun;4(6):815–818. doi: 10.1097/00001756-199306000-00055. [DOI] [PubMed] [Google Scholar]
- Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996 Aug;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Alzheimer's disease: genotypes, phenotypes, and treatments. Science. 1997 Jan 31;275(5300):630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., Kim G., Ratovitsky T., Davenport F., Nordstedt C., Seeger M. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996 Jul;17(1):181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Weidemann A., Paliga K., Dürrwang U., Czech C., Evin G., Masters C. L., Beyreuther K. Formation of stable complexes between two Alzheimer's disease gene products: presenilin-2 and beta-amyloid precursor protein. Nat Med. 1997 Mar;3(3):328–332. doi: 10.1038/nm0397-328. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Castaño E. M., Golabek A., Vogel T., Frangione B. Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994 Nov;145(5):1030–1035. [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Dowjat W. K., Permanne B., Palha J., Kumar A., Gallo G., Frangione B. Presenilin-1 is associated with Alzheimer's disease amyloid. Am J Pathol. 1997 Aug;151(2):601–610. [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992 Feb 3;135(2):235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Palha J. A., Ghiso J., Frangione B. S182 protein in Alzheimer's disease neuritic plaques. Lancet. 1995 Nov 18;346(8986):1366–1366. doi: 10.1016/s0140-6736(95)92379-9. [DOI] [PubMed] [Google Scholar]
- Xia W., Zhang J., Perez R., Koo E. H., Selkoe D. J. Interaction between amyloid precursor protein and presenilins in mammalian cells: implications for the pathogenesis of Alzheimer disease. Proc Natl Acad Sci U S A. 1997 Jul 22;94(15):8208–8213. doi: 10.1073/pnas.94.15.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]