Abstract
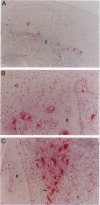
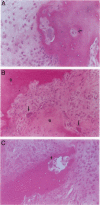
Focal resorption of bone at the bone-pannus interface is common in rheumatoid arthritis (RA) and juvenile rheumatoid arthritis (JRA) and can result in significant morbidity. However, the specific cellular and hormonal mechanisms involved in this process are not well established. We examined tissue sections from areas of bone erosion in patients with RA and JRA. Multinucleated cells (MNCs) were present in resorption lacunae in areas of calcified cartilage and in subchondral bone immediately adjacent to calcified cartilage, as previously described. mRNA for the calcitonin receptor (CTR) was localized to these MNCs in bone resorption lacunae, a finding that definitively identifies these cells as osteoclasts. These MNCs were also positive for tartrate-resistant acid phosphatase (TRAP) mRNA and TRAP enzymatic activity. Occasional mononuclear cells on the bone surface were also CTR positive. Mononuclear cells and MNCs not on bone surfaces were CTR negative. The restriction of CTR-positive cells to the surface of mineralized tissues suggests that bone and/or calcified cartilage provide signals that are critical for the differentiation of hematopoietic osteoclast precursors to fully differentiated osteoclasts. Some MNCs and mononuclear cells off bone and within invading tissues were TRAP positive. These cells likely represent the precursors of the CTR-TRAP-positive cells on bone. Parathyroid hormone receptor mRNA was present in cells with the phenotypic appearance of osteoblasts, in close proximity to MNCs, and in occasional cells within pannus tissue, but not in the MNCs in bone resorption lacunae. These findings demonstrate that osteoclasts within the rheumatoid lesion do not express parathyroid hormone receptor. In conclusion, the resorbing cells in RA exhibit a definitive osteoclastic phenotype, suggesting that pharmacological agents that inhibit osteoclast recruitment or activity are rational targets for blocking focal bone erosion in patients with RA and JRA.
Full text
PDF


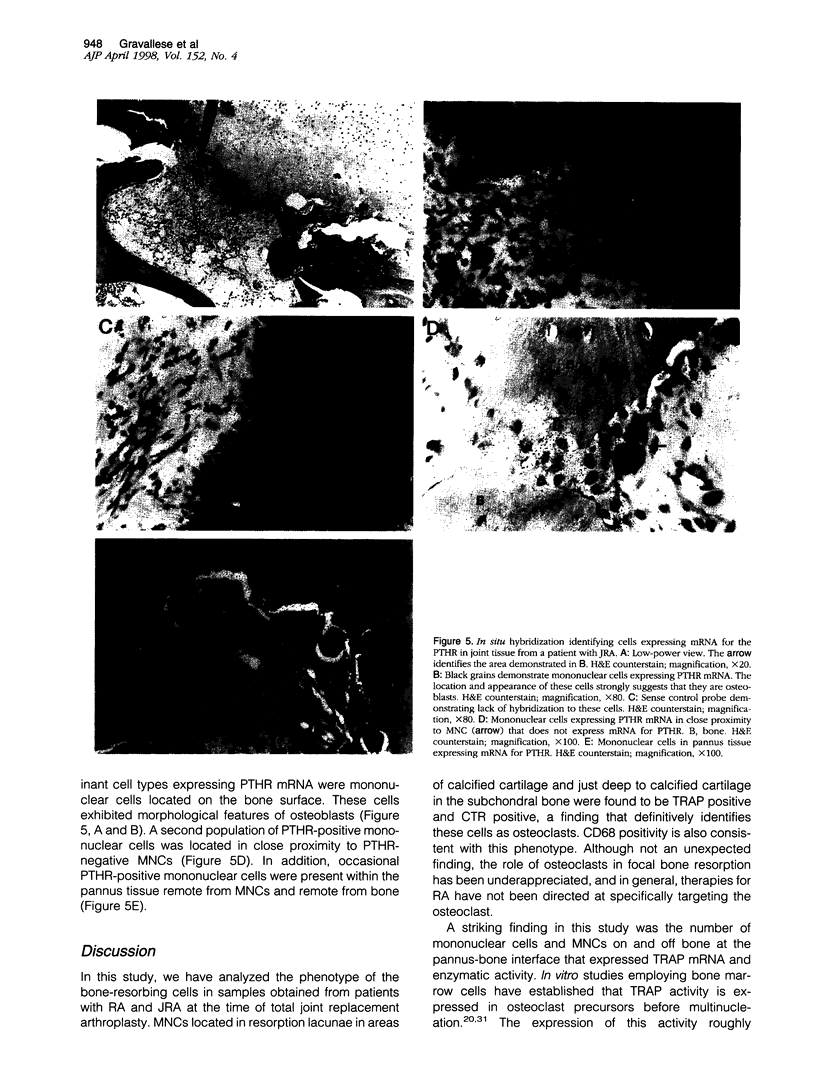



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwala N., Gay C. V. Specific binding of parathyroid hormone to living osteoclasts. J Bone Miner Res. 1992 May;7(5):531–539. doi: 10.1002/jbmr.5650070509. [DOI] [PubMed] [Google Scholar]
- Ashton B. A., Ashton I. K., Marshall M. J., Butler R. C. Localisation of vitronectin receptor immunoreactivity and tartrate resistant acid phosphatase activity in synovium from patients with inflammatory or degenerative arthritis. Ann Rheum Dis. 1993 Feb;52(2):133–137. doi: 10.1136/ard.52.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P., Costantini M., Dearden L. C., Bonucci E. Expression of tartrate-resistant acid phosphatase in bone marrow macrophages. Basic Appl Histochem. 1987;31(4):433–440. [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984 Sep;27(9):968–975. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- Burkhart J. M., Jowsey J. Parathyroid and thyroid hormones in the development of immobilization osteoporosis. Endocrinology. 1967 Nov;81(5):1053–1062. doi: 10.1210/endo-81-5-1053. [DOI] [PubMed] [Google Scholar]
- Chang J. S., Quinn J. M., Demaziere A., Bulstrode C. J., Francis M. J., Duthie R. B., Athanasou N. A. Bone resorption by cells isolated from rheumatoid synovium. Ann Rheum Dis. 1992 Nov;51(11):1223–1229. doi: 10.1136/ard.51.11.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Allard S., Abney E., Feldmann M., Maini R. N. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol. 1992 Oct;31(10):653–661. doi: 10.1093/rheumatology/31.10.653. [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Feldmann M., Maini R. N. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991 Sep;34(9):1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- Compston J. E., Vedi S., Croucher P. I., Garrahan N. J., O'Sullivan M. M. Bone turnover in non-steroid treated rheumatoid arthritis. Ann Rheum Dis. 1994 Mar;53(3):163–166. doi: 10.1136/ard.53.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp A. J., Helliwell M., Grahame R. The effect of hyperparathyroidism on the course of rheumatoid arthritis. Br J Rheumatol. 1983 Feb;22(1):22–28. doi: 10.1093/rheumatology/22.1.22. [DOI] [PubMed] [Google Scholar]
- Darling J. M., Goldring S. R., Harada Y., Handel M. L., Glowacki J., Gravallese E. M. Multinucleated cells in pigmented villonodular synovitis and giant cell tumor of tendon sheath express features of osteoclasts. Am J Pathol. 1997 Apr;150(4):1383–1393. [PMC free article] [PubMed] [Google Scholar]
- Deleuran B. W., Chu C. Q., Field M., Brennan F. M., Katsikis P., Feldmann M., Maini R. N. Localization of interleukin-1 alpha, type 1 interleukin-1 receptor and interleukin-1 receptor antagonist in the synovial membrane and cartilage/pannus junction in rheumatoid arthritis. Br J Rheumatol. 1992 Dec;31(12):801–809. doi: 10.1093/rheumatology/31.12.801. [DOI] [PubMed] [Google Scholar]
- Dewhirst F. E., Ago J. M., Peros W. J., Stashenko P. Synergism between parathyroid hormone and interleukin 1 in stimulating bone resorption in organ culture. J Bone Miner Res. 1987 Apr;2(2):127–134. doi: 10.1002/jbmr.5650020208. [DOI] [PubMed] [Google Scholar]
- Fujikawa Y., Sabokbar A., Neale S., Athanasou N. A. Human osteoclast formation and bone resorption by monocytes and synovial macrophages in rheumatoid arthritis. Ann Rheum Dis. 1996 Nov;55(11):816–822. doi: 10.1136/ard.55.11.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa Y., Shingu M., Torisu T., Itonaga I., Masumi S. Bone resorption by tartrate-resistant acid phosphatase-positive multinuclear cells isolated from rheumatoid synovium. Br J Rheumatol. 1996 Mar;35(3):213–217. doi: 10.1093/rheumatology/35.3.213. [DOI] [PubMed] [Google Scholar]
- Glowacki J., Jasty M., Goldring S. Comparison of multinucleated cells elicited in rats by particulate bone, polyethylene, or polymethylmethacrylate. J Bone Miner Res. 1986 Aug;1(4):327–331. doi: 10.1002/jbmr.5650010405. [DOI] [PubMed] [Google Scholar]
- Glowacki J., Rey C., Glimcher M. J., Cox K. A., Lian J. A role for osteocalcin in osteoclast differentiation. J Cell Biochem. 1991 Mar;45(3):292–302. doi: 10.1002/jcb.240450312. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Roelke M., Glowacki J. Multinucleated cells elicited in response to implants of devitalized bone particles possess receptors for calcitonin. J Bone Miner Res. 1988 Feb;3(1):117–120. doi: 10.1002/jbmr.5650030118. [DOI] [PubMed] [Google Scholar]
- Gorn A. H., Lin H. Y., Yamin M., Auron P. E., Flannery M. R., Tapp D. R., Manning C. A., Lodish H. F., Krane S. M., Goldring S. R. Cloning, characterization, and expression of a human calcitonin receptor from an ovarian carcinoma cell line. J Clin Invest. 1992 Nov;90(5):1726–1735. doi: 10.1172/JCI116046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough A. K., Peel N. F., Eastell R., Holder R. L., Lilley J., Emery P. Excretion of pyridinium crosslinks correlates with disease activity and appendicular bone loss in early rheumatoid arthritis. Ann Rheum Dis. 1994 Jan;53(1):14–17. doi: 10.1136/ard.53.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda Y., Hiura K., Sato T., Okazaki R., Matsumoto T., Ogata E., Ishitani R., Kumegawa M. Existence of parathyroid hormone binding sites on murine hemopoietic blast cells. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1481–1486. doi: 10.1016/0006-291x(89)91146-7. [DOI] [PubMed] [Google Scholar]
- Hall G. M., Spector T. D., Delmas P. D. Markers of bone metabolism in postmenopausal women with rheumatoid arthritis. Effects of corticosteroids and hormone replacement therapy. Arthritis Rheum. 1995 Jul;38(7):902–906. doi: 10.1002/art.1780380705. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology. 1989 Sep;125(3):1606–1612. doi: 10.1210/endo-125-3-1606. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Generation of osteoclastic function in mouse bone marrow cultures: multinuclearity and tartrate-resistant acid phosphatase are unreliable markers for osteoclastic differentiation. Endocrinology. 1989 Apr;124(4):1689–1696. doi: 10.1210/endo-124-4-1689. [DOI] [PubMed] [Google Scholar]
- Horton M. Vitronectin receptor: tissue specific expression or adaptation to culture? Int J Exp Pathol. 1990 Oct;71(5):741–759. [PMC free article] [PubMed] [Google Scholar]
- Ikegame M., Rakopoulos M., Zhou H., Houssami S., Martin T. J., Moseley J. M., Findlay D. M. Calcitonin receptor isoforms in mouse and rat osteoclasts. J Bone Miner Res. 1995 Jan;10(1):59–65. doi: 10.1002/jbmr.5650100110. [DOI] [PubMed] [Google Scholar]
- Kaji H., Sugimoto T., Kanatani M., Miyauchi A., Kimura T., Sakakibara S., Fukase M., Chihara K. Carboxyl-terminal parathyroid hormone fragments stimulate osteoclast-like cell formation and osteoclastic activity. Endocrinology. 1994 Apr;134(4):1897–1904. doi: 10.1210/endo.134.4.8137758. [DOI] [PubMed] [Google Scholar]
- Kotake S., Sato K., Kim K. J., Takahashi N., Udagawa N., Nakamura I., Yamaguchi A., Kishimoto T., Suda T., Kashiwazaki S. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996 Jan;11(1):88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- Kröger H., Risteli J., Risteli L., Penttilä I., Alhava E. Serum osteocalcin and carboxyterminal propeptide of type I procollagen in rheumatoid arthritis. Ann Rheum Dis. 1993 May;52(5):338–342. doi: 10.1136/ard.52.5.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. K., Goldring S. R., Lorenzo J. A. Expression of the calcitonin receptor in bone marrow cell cultures and in bone: a specific marker of the differentiated osteoclast that is regulated by calcitonin. Endocrinology. 1995 Oct;136(10):4572–4581. doi: 10.1210/endo.136.10.7664679. [DOI] [PubMed] [Google Scholar]
- Leisen J. C., Duncan H., Riddle J. M., Pitchford W. C. The erosive front: a topographic study of the junction between the pannus and the subchondral plate in the macerated rheumatoid metacarpal head. J Rheumatol. 1988 Jan;15(1):17–22. [PubMed] [Google Scholar]
- Lin H. Y., Harris T. L., Flannery M. S., Aruffo A., Kaji E. H., Gorn A., Kolakowski L. F., Jr, Lodish H. F., Goldring S. R. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science. 1991 Nov 15;254(5034):1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- Mazess R. B., Whedon G. D. Immobilization and bone. Calcif Tissue Int. 1983 May;35(3):265–267. doi: 10.1007/BF02405043. [DOI] [PubMed] [Google Scholar]
- Mellish R. W., O'Sullivan M. M., Garrahan N. J., Compston J. E. Iliac crest trabecular bone mass and structure in patients with non-steroid treated rheumatoid arthritis. Ann Rheum Dis. 1987 Nov;46(11):830–836. doi: 10.1136/ard.46.11.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook P. N., Ansell B. M., Foster S., Gumpel J. M., Hesp R., Reeve J., Zanelli J. M. Bone turnover in early rheumatoid arthritis. 1. Biochemical and kinetic indexes. Ann Rheum Dis. 1985 Sep;44(9):575–579. doi: 10.1136/ard.44.9.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook P. N., Shawe D., Hesp R., Zanelli J. M., Mitchell R., Katz D., Gumpel J. M., Ansell B. M., Reeve J. Rapid periarticular bone loss in rheumatoid arthritis. Possible promotion by normal circulating concentrations of parathyroid hormone or calcitriol (1,25-dihydroxyvitamin D3). Arthritis Rheum. 1990 May;33(5):615–622. doi: 10.1002/art.1780330503. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Shiozawa S., Shiozawa K., Imura S., Fujita T. Quantitative histologic studies on the pathogenesis of periarticular osteoporosis in rheumatoid arthritis. Arthritis Rheum. 1985 Jan;28(1):25–31. doi: 10.1002/art.1780280105. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi N., Martin T. J. Modulation of osteoclast differentiation. Endocr Rev. 1992 Feb;13(1):66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Kanatani M., Kaji H., Yamaguchi T., Fukase M., Chihara K. Second messenger signaling of PTH- and PTHRP-stimulated osteoclast-like cell formation from hemopoietic blast cells. Am J Physiol. 1993 Sep;265(3 Pt 1):E367–E373. doi: 10.1152/ajpendo.1993.265.3.E367. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Akatsu T., Sasaki T., Nicholson G. C., Moseley J. M., Martin T. J., Suda T. Induction of calcitonin receptors by 1 alpha, 25-dihydroxyvitamin D3 in osteoclast-like multinucleated cells formed from mouse bone marrow cells. Endocrinology. 1988 Sep;123(3):1504–1510. doi: 10.1210/endo-123-3-1504. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Goldring S., Katz M., Hilsenbeck S., Williams R., Roodman G. D. Downregulation of calcitonin receptor mRNA expression by calcitonin during human osteoclast-like cell differentiation. J Clin Invest. 1995 Jan;95(1):167–171. doi: 10.1172/JCI117634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti A., Rizzoli R., Zambonin Zallone A. Parathyroid hormone binding to cultured avian osteoclasts. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1217–1222. doi: 10.1016/0006-291x(91)91551-m. [DOI] [PubMed] [Google Scholar]
- Tetlow L. C., Woolley D. E. Mast cells, cytokines, and metalloproteinases at the rheumatoid lesion: dual immunolocalisation studies. Ann Rheum Dis. 1995 Nov;54(11):896–903. doi: 10.1136/ard.54.11.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabandt A., Gay R. E., Fassbender H. G., Gay S. Cathepsin B in synovial cells at the site of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1991 Nov;34(11):1444–1451. doi: 10.1002/art.1780341116. [DOI] [PubMed] [Google Scholar]
- Wilkinson L. S., Pitsillides A. A., Edwards J. C. Giant cells in arthritic synovium. Ann Rheum Dis. 1993 Mar;52(3):182–184. doi: 10.1136/ard.52.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Crossley M. J., Evanson J. M. Collagenase at sites of cartilage erosion in the rheumatoid joint. Arthritis Rheum. 1977 Jul-Aug;20(6):1231–1239. doi: 10.1002/art.1780200612. [DOI] [PubMed] [Google Scholar]
- van de Wijngaert F. P., Burger E. H. Demonstration of tartrate-resistant acid phosphatase in un-decalcified, glycolmethacrylate-embedded mouse bone: a possible marker for (pre)osteoclast identification. J Histochem Cytochem. 1986 Oct;34(10):1317–1323. doi: 10.1177/34.10.3745910. [DOI] [PubMed] [Google Scholar]