Abstract
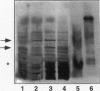
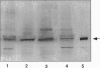
Oxygen radicals and oxidatively modified proteins seem to participate in degenerative vascular and inflammatory diseases. Factors that contribute to the development of atherosclerosis, eg, oxidation of low-density lipoproteins (LDLs), may also contribute to glomerulosclerosis. Although the nature of the in vivo oxidants remains unknown, recent findings indicated that the myeloperoxidase (MPO)-H2O2-halide system could play an important role in modification of (lipo)proteins in human tissues. MPO, the enzyme responsible for hypochlorite (HOCl/OCl-) formation, is present in human atherosclerotic lesions and in inflammatory conditions. In the present study, MPO was identified by Western blot analysis and immunohistochemical technique in diseased human kidney either with primarily sclerotic or inflammatory lesions. Furthermore, the presence of HOCl-modified proteins was demonstrated in diseased renal tissues using a specific monoclonal antibody (clone 2D10G9), raised against HOCl-modified LDL, that does not cross-react with native LDL or Cu(2+)-, 4-hydroxynonenal-, or malondialdehyde-modified LDL. The antibody recognized HOCl-modified proteins in glomerular and tubulointerstitial inflammatory and fibrotic lesions and pronounced immunostaining was demonstrated in mononuclear cells. LDL or human serum albumin oxidized by HOCl in vitro, but not native LDL or human serum albumin, effectively competed with epitopes in diseased kidney for antibody binding. Western blot analysis in diseased kidney protein samples revealed at least two major proteins recognized by the anti-HOCl-modified protein monoclonal antibody. Densitometric evaluation of immunoreactive bands obtained under these conditions demonstrated that expression of HOCl-modified proteins is tightly coupled to expression of immunoreactive MPO in the same tissue samples. From our studies it is proposed that oxidation of proteins by HOCl might be a leading event in glomerular and tubulointerstitial injury. By this mechanism, mononuclear cells, a permanent source for MPO, may play a key role in the development of nephrosclerosis, glomerulo-clerosis, and tubulointerstitial fibrosis, respectively.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Baker P. J., Johnson R. J., Ochi R. F., Pritzl P., Couser W. G. Complement membrane attack complex stimulates production of reactive oxygen metabolites by cultured rat mesangial cells. J Clin Invest. 1986 Mar;77(3):762–767. doi: 10.1172/JCI112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud L., Ardaillou R. Reactive oxygen species: production and role in the kidney. Am J Physiol. 1986 Nov;251(5 Pt 2):F765–F776. doi: 10.1152/ajprenal.1986.251.5.F765. [DOI] [PubMed] [Google Scholar]
- Berliner J. A., Heinecke J. W. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20(5):707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- Boyd H. C., Gown A. M., Wolfbauer G., Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol. 1989 Nov;135(5):815–825. [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G. Cellular injury by oxidants. Am J Med. 1991 Sep 30;91(3C):23S–30S. doi: 10.1016/0002-9343(91)90280-b. [DOI] [PubMed] [Google Scholar]
- Coritsidis G., Rifici V., Gupta S., Rie J., Shan Z. H., Neugarten J., Schlondorff D. Preferential binding of oxidized LDL to rat glomeruli in vivo and cultured mesangial cells in vitro. Kidney Int. 1991 May;39(5):858–866. doi: 10.1038/ki.1991.108. [DOI] [PubMed] [Google Scholar]
- Daugherty A., Dunn J. L., Rateri D. L., Heinecke J. W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994 Jul;94(1):437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. R., Karnovsky M. J. Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int. 1988 May;33(5):917–924. doi: 10.1038/ki.1988.87. [DOI] [PubMed] [Google Scholar]
- Gröne E. F., Abboud H. E., Höhne M., Walli A. K., Gröne H. J., Stüker D., Robenek H., Wieland E., Seidel D. Actions of lipoproteins in cultured human mesangial cells: modulation by mitogenic vasoconstrictors. Am J Physiol. 1992 Oct;263(4 Pt 2):F686–F696. doi: 10.1152/ajprenal.1992.263.4.F686. [DOI] [PubMed] [Google Scholar]
- Gröne E. F., Walli A. K., Gröne H. J., Miller B., Seidel D. The role of lipids in nephrosclerosis and glomerulosclerosis. Atherosclerosis. 1994 May;107(1):1–13. doi: 10.1016/0021-9150(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Gröne H. J., Walli A. K., Gröne E., Krämer A., Clemens M. R., Seidel D. Receptor mediated uptake of apo B and apo E rich lipoproteins by human glomerular epithelial cells. Kidney Int. 1990 Jun;37(6):1449–1459. doi: 10.1038/ki.1990.135. [DOI] [PubMed] [Google Scholar]
- Gröne H. J., Walli A., Gröne E., Niedmann P., Thiery J., Seidel D., Helmchen U. Induction of glomerulosclerosis by dietary lipids. A functional and morphologic study in the rat. Lab Invest. 1989 Mar;60(3):433–446. [PubMed] [Google Scholar]
- Gröne H. J., Weber K., Gröne E., Helmchen U., Osborn M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol. 1987 Oct;129(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Rifici V., Crowley S., Brownlee M., Shan Z., Schlondorff D. Interactions of LDL and modified LDL with mesangial cells and matrix. Kidney Int. 1992 May;41(5):1161–1169. doi: 10.1038/ki.1992.177. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hazell L. J., Arnold L., Flowers D., Waeg G., Malle E., Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996 Mar 15;97(6):1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell L. J., Stocker R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem J. 1993 Feb 15;290(Pt 1):165–172. doi: 10.1042/bj2900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell L. J., van den Berg J. J., Stocker R. Oxidation of low-density lipoprotein by hypochlorite causes aggregation that is mediated by modification of lysine residues rather than lipid oxidation. Biochem J. 1994 Aug 15;302(Pt 1):297–304. doi: 10.1042/bj3020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillegass L. M., Griswold D. E., Brickson B., Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990 Dec;24(4):285–295. doi: 10.1016/0160-5402(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Holvoet P., Perez G., Zhao Z., Brouwers E., Bernar H., Collen D. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J Clin Invest. 1995 Jun;95(6):2611–2619. doi: 10.1172/JCI117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen Y. M., Van Houten B., Borm P. J., Mossman B. T. Cell and tissue responses to oxidative damage. Lab Invest. 1993 Sep;69(3):261–274. [PubMed] [Google Scholar]
- Johnson R. J., Couser W. G., Chi E. Y., Adler S., Klebanoff S. J. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1987 May;79(5):1379–1387. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Guggenheim S. J., Klebanoff S. J., Ochi R. F., Wass A., Baker P., Schulze M., Couser W. G. Morphologic correlates of glomerular oxidant injury induced by the myeloperoxidase-hydrogen peroxide-halide system of the neutrophil. Lab Invest. 1988 Mar;58(3):294–301. [PubMed] [Google Scholar]
- Johnson R. J., Klebanoff S. J., Ochi R. F., Adler S., Baker P., Sparks L., Couser W. G. Participation of the myeloperoxidase-H2O2-halide system in immune complex nephritis. Kidney Int. 1987 Sep;32(3):342–349. doi: 10.1038/ki.1987.215. [DOI] [PubMed] [Google Scholar]
- Jürgens G., Chen Q., Esterbauer H., Mair S., Ledinski G., Dinges H. P. Immunostaining of human autopsy aortas with antibodies to modified apolipoprotein B and apoprotein(a). Arterioscler Thromb. 1993 Nov;13(11):1689–1699. doi: 10.1161/01.atv.13.11.1689. [DOI] [PubMed] [Google Scholar]
- Keane W. F. Lipids and the kidney. Kidney Int. 1994 Sep;46(3):910–920. doi: 10.1038/ki.1994.349. [DOI] [PubMed] [Google Scholar]
- Keane W. F., O'Donnell M. P., Kasiske B. L., Kim Y. Oxidative modification of low-density lipoproteins by mesangial cells. J Am Soc Nephrol. 1993 Aug;4(2):187–194. doi: 10.1681/ASN.V42187. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Kinsella M. G., Wight T. N. Degradation of endothelial cell matrix heparan sulfate proteoglycan by elastase and the myeloperoxidase-H2O2-chloride system. Am J Pathol. 1993 Sep;143(3):907–917. [PMC free article] [PubMed] [Google Scholar]
- Kotani K., Maekawa M., Kanno T., Kondo A., Toda N., Manabe M. Distribution of immunoreactive malondialdehyde-modified low-density lipoprotein in human serum. Biochim Biophys Acta. 1994 Nov 17;1215(1-2):121–125. doi: 10.1016/0005-2760(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Laight D. W., Lad N., Woodward B., Waterfall J. F. Assessment of myeloperoxidase activity in renal tissue after ischemia/reperfusion. Eur J Pharmacol. 1994 Nov 1;292(1):81–88. doi: 10.1016/0926-6917(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Koh H. I. Visualization of binding and uptake of oxidized low density lipoproteins by cultured mesangial cells. Lab Invest. 1994 Aug;71(2):200–208. [PubMed] [Google Scholar]
- Maggi E., Bellazzi R., Falaschi F., Frattoni A., Perani G., Finardi G., Gazo A., Nai M., Romanini D., Bellomo G. Enhanced LDL oxidation in uremic patients: an additional mechanism for accelerated atherosclerosis? Kidney Int. 1994 Mar;45(3):876–883. doi: 10.1038/ki.1994.115. [DOI] [PubMed] [Google Scholar]
- Maggi E., Bellazzi R., Gazo A., Seccia M., Bellomo G. Autoantibodies against oxidatively-modified LDL in uremic patients undergoing dialysis. Kidney Int. 1994 Sep;46(3):869–876. doi: 10.1038/ki.1994.344. [DOI] [PubMed] [Google Scholar]
- Magil A. B., Frohlich J. J., Innis S. M., Steinbrecher U. P. Oxidized low-density lipoprotein in experimental focal glomerulosclerosis. Kidney Int. 1993 Jun;43(6):1243–1250. doi: 10.1038/ki.1993.176. [DOI] [PubMed] [Google Scholar]
- Malle E., Hazell L., Stocker R., Sattler W., Esterbauer H., Waeg G. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler Thromb Vasc Biol. 1995 Jul;15(7):982–989. doi: 10.1161/01.atv.15.7.982. [DOI] [PubMed] [Google Scholar]
- Malle E., Ibovnik A., Stienmetz A., Kostner G. M., Sattler W. Identification of glycoprotein IIb as the lipoprotein(a)-binding protein on platelets. Lipoprotein(a) binding is independent of an arginyl-glycyl-aspartate tripeptide located in apolipoprotein(a). Arterioscler Thromb. 1994 Mar;14(3):345–352. doi: 10.1161/01.atv.14.3.345. [DOI] [PubMed] [Google Scholar]
- Neale T. J., Ojha P. P., Exner M., Poczewski H., Rüger B., Witztum J. L., Davis P., Kerjaschki D. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Invest. 1994 Oct;94(4):1577–1584. doi: 10.1172/JCI117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y., Kasama T., Miyachi Y., Kanoh T. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: cross-sectional and longitudinal studies. Life Sci. 1989;44(22):1655–1664. doi: 10.1016/0024-3205(89)90482-7. [DOI] [PubMed] [Google Scholar]
- O'Connell A. M., Gieseg S. P., Stanley K. K. Hypochlorite oxidation causes cross-linking of Lp(a). Biochim Biophys Acta. 1994 Jan 11;1225(2):180–186. doi: 10.1016/0925-4439(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Ong A. C., Moorhead J. F. Tubular lipidosis: epiphenomenon or pathogenetic lesion in human renal disease? Kidney Int. 1994 Mar;45(3):753–762. doi: 10.1038/ki.1994.100. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Khoo J. C., Miller E., Parthasarathy S., Palinski W., Witztum J. L. Macrophage-derived foam cells freshly isolated from rabbit atherosclerotic lesions degrade modified lipoproteins, promote oxidation of low-density lipoproteins, and contain oxidation-specific lipid-protein adducts. J Clin Invest. 1991 Jan;87(1):90–99. doi: 10.1172/JCI115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Rovin B. H., Wurst E., Kohan D. E. Production of reactive oxygen species by tubular epithelial cells in culture. Kidney Int. 1990 Jun;37(6):1509–1514. doi: 10.1038/ki.1990.142. [DOI] [PubMed] [Google Scholar]
- Ruiz-Torres P., Gonzalez-Rubio M., Lucio-Cazaña F. J., Ruiz-Villaespesa A., Rodriguez-Puyol M., Rodriguez-Puyol D. Reactive oxygen species and platelet-activating factor synthesis in age-related glomerulosclerosis. J Lab Clin Med. 1994 Oct;124(4):489–495. [PubMed] [Google Scholar]
- Salonen J. T., Ylä-Herttuala S., Yamamoto R., Butler S., Korpela H., Salonen R., Nyyssönen K., Palinski W., Witztum J. L. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992 Apr 11;339(8798):883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- Savenkova M. L., Mueller D. M., Heinecke J. W. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem. 1994 Aug 12;269(32):20394–20400. [PubMed] [Google Scholar]
- Schierwagen C., Bylund-Fellenius A. C., Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990 May;23(3):179–186. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- Springall D. R., Hacker G. W., Grimelius L., Polak J. M. The potential of the immunogold-silver staining method for paraffin sections. Histochemistry. 1984;81(6):603–608. doi: 10.1007/BF00489542. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Zhang H. F., Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9(2):155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- Sutherland W. H., Walker R. J., Ball M. J., Stapley S. A., Robertson M. C. Oxidation of low density lipoproteins from patients with renal failure or renal transplants. Kidney Int. 1995 Jul;48(1):227–236. doi: 10.1038/ki.1995.288. [DOI] [PubMed] [Google Scholar]
- Van Goor H., Ding G., Kees-Folts D., Grond J., Schreiner G. F., Diamond J. R. Macrophages and renal disease. Lab Invest. 1994 Oct;71(4):456–464. [PubMed] [Google Scholar]
- Vissers M. C., Winterbourn C. C., Hunt J. S. Degradation of glomerular basement membrane by human neutrophils in vitro. Biochim Biophys Acta. 1984 Jun 19;804(2):154–160. doi: 10.1016/0167-4889(84)90144-7. [DOI] [PubMed] [Google Scholar]
- Vissers M. C., Winterbourn C. C. The effect of oxidants on neutrophil-mediated degradation of glomerular basement membrane collagen. Biochim Biophys Acta. 1986 Dec 19;889(3):277–286. doi: 10.1016/0167-4889(86)90190-4. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]
- Wheeler D. C., Chana R. S., Topley N., Petersen M. M., Davies M., Williams J. D. Oxidation of low density lipoprotein by mesangial cells may promote glomerular injury. Kidney Int. 1994 Jun;45(6):1628–1636. doi: 10.1038/ki.1994.214. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985 Jun 18;840(2):204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- Yamada T., Grisham M. B. Role of neutrophil-derived oxidants in the pathogenesis of intestinal inflammation. Klin Wochenschr. 1991 Dec 15;69(21-23):988–994. doi: 10.1007/BF01645144. [DOI] [PubMed] [Google Scholar]
- van Goor H., Fidler V., Weening J. J., Grond J. Determinants of focal and segmental glomerulosclerosis in the rat after renal ablation. Evidence for involvement of macrophages and lipids. Lab Invest. 1991 Jun;64(6):754–765. [PubMed] [Google Scholar]