Abstract
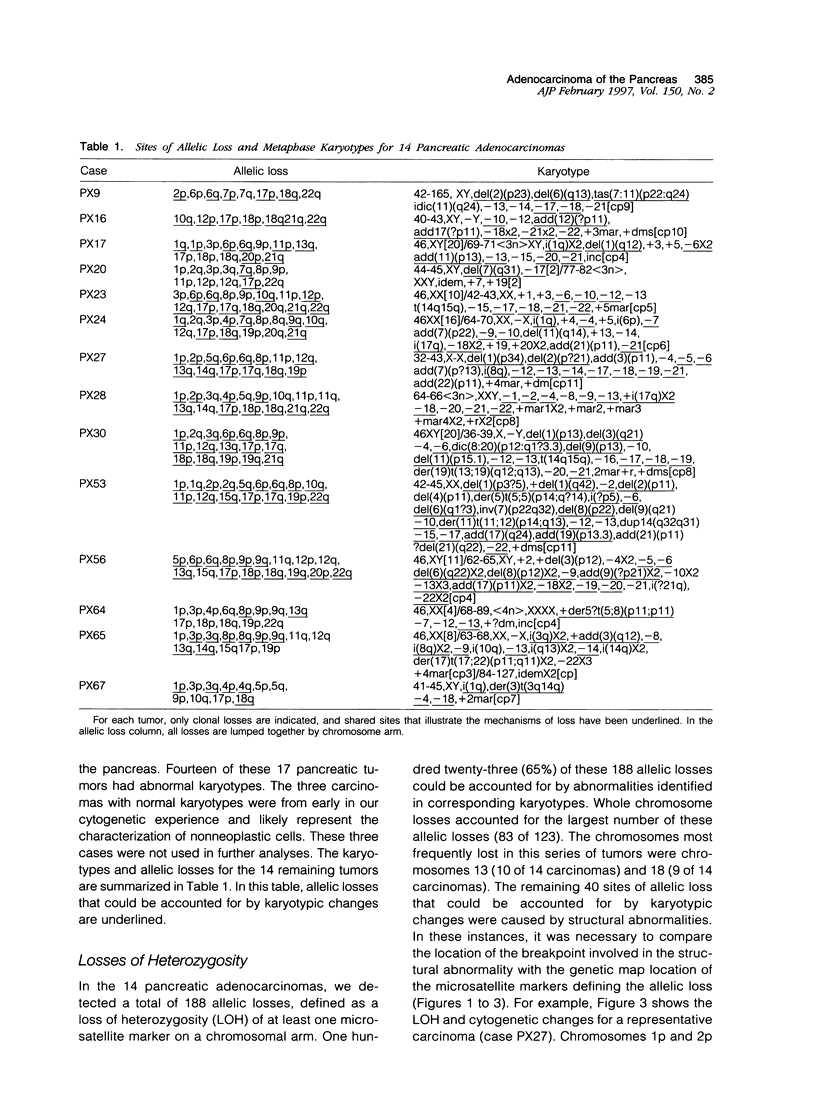
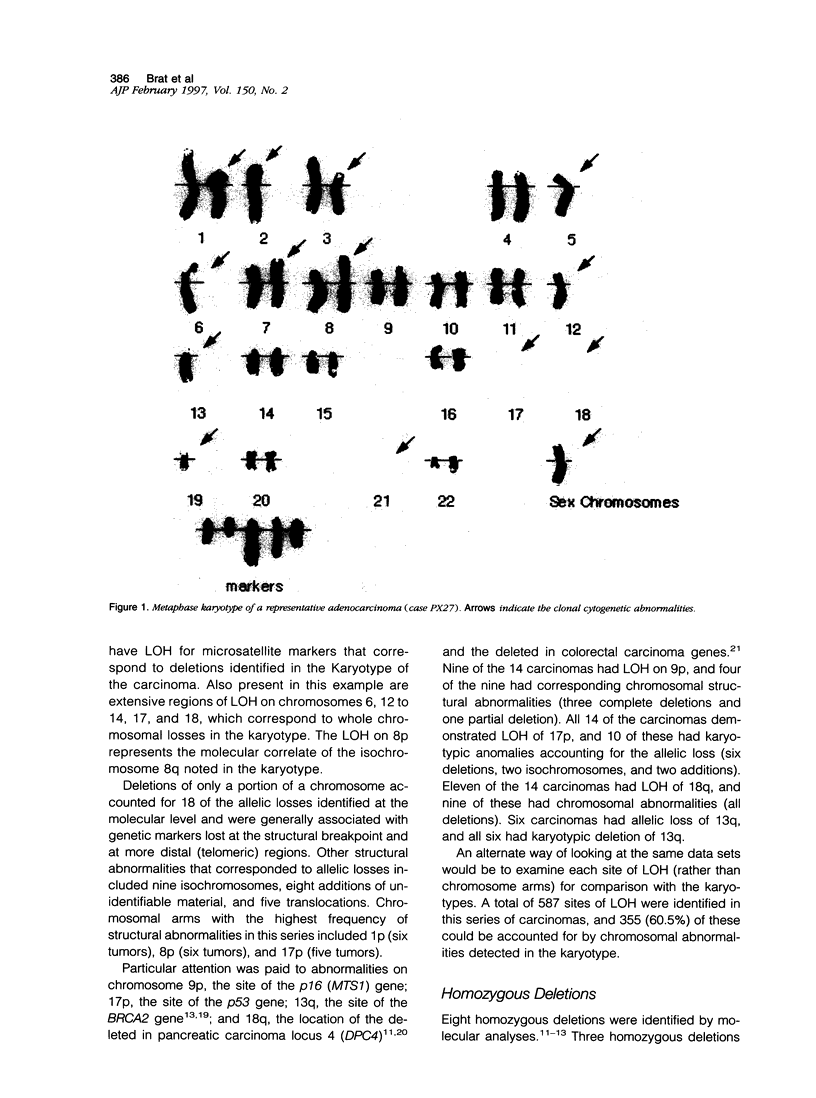
Molecular genetic alterations are known to be important in human carcinoma, but the structural basis of these changes is largely unknown. To examine the basis of these changes, we compared the karyotypic chromosomal abnormalities of primary pancreatic adenocarcinomas with the molecular changes identified in these same cancers. In 14 cancers with abnormal karyotypes, 65% (123 of 188) of the chromosomal arms with molecular loss of heterozygosity (LOH) were associated with karyotypic structural anomalies. Karyotypic changes accounting for these molecular allelic losses included 83 chromosome losses, 18 partial deletions, nine isochromosomes, eight additions, and five translocations. Eight bomozygous deletions were also identified by molecular analyses. Of the three homozygous deletions identified at 9p21, the only karyotypic change was a single case in which one entire copy of chromosome 9 was deleted. Of the four homozygous deletions identified at 18q21.1, one showed a loss of both copies of chromosome 18, two showed a loss of one copy of chromosome 18, and the fourth had two structurally normal copies of chromosome 18. One homozygous deletion was identified at 13q12.3, and the karyotype revealed the loss of one entire copy of chromosome 13. The second copy of chromosome 13 in this carcinoma was structurally normal. These results indicate that chromosomal structural anomalies can account for two-thirds of the LOH in pancreatic adenocarcinomas and that most homozygous deletions are likely to be interstitial chromosomal deletions that are below the detection limit of conventional karyotypic analyses. Some of the molecular deletions detected as LOH on chromosomes with karyotypically normal structure can be explained by chromosomal loss with reduplication of the remaining chromosome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bello M. J., de Campos J. M., Vaquero J., Kusak M. E., Sarasa J. L., Pestaña A., Rey J. A. Molecular and cytogenetic analysis of chromosome 9 deletions in 75 malignant gliomas. Genes Chromosomes Cancer. 1994 Jan;9(1):33–41. doi: 10.1002/gcc.2870090107. [DOI] [PubMed] [Google Scholar]
- Biegel J. A., Burk C. D., Parmiter A. H., Emanuel B. S. Molecular analysis of a partial deletion of 22q in a central nervous system rhabdoid tumor. Genes Chromosomes Cancer. 1992 Sep;5(2):104–108. doi: 10.1002/gcc.2870050203. [DOI] [PubMed] [Google Scholar]
- Buetow K. H., Weber J. L., Ludwigsen S., Scherpbier-Heddema T., Duyk G. M., Sheffield V. C., Wang Z., Murray J. C. Integrated human genome-wide maps constructed using the CEPH reference panel. Nat Genet. 1994 Apr;6(4):391–393. doi: 10.1038/ng0494-391. [DOI] [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., Hruban R. H., Redston M. S., Yeo C. J., Kern S. E. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res. 1994 Jul 1;54(13):3568–3573. [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- De Sepulveda P., Guenet J. L., Panthier J. J. Phenotypic reversions at the W/Kit locus mediated by mitotic recombination in mice. Mol Cell Biol. 1995 Nov;15(11):5898–5905. doi: 10.1128/mcb.15.11.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiuseppe J. A., Hruban R. H., Offerhaus G. J., Clement M. J., van den Berg F. M., Cameron J. L., van Mansfeld A. D. Detection of K-ras mutations in mucinous pancreatic duct hyperplasia from a patient with a family history of pancreatic carcinoma. Am J Pathol. 1994 May;144(5):889–895. [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Griffin C. A., Hruban R. H., Morsberger L. A., Ellingham T., Long P. P., Jaffee E. M., Hauda K. M., Bohlander S. K., Yeo C. J. Consistent chromosome abnormalities in adenocarcinoma of the pancreas. Cancer Res. 1995 Jun 1;55(11):2394–2399. [PubMed] [Google Scholar]
- Hahn S. A., Hoque A. T., Moskaluk C. A., da Costa L. T., Schutte M., Rozenblum E., Seymour A. B., Weinstein C. L., Yeo C. J., Hruban R. H. Homozygous deletion map at 18q21.1 in pancreatic cancer. Cancer Res. 1996 Feb 1;56(3):490–494. [PubMed] [Google Scholar]
- Hahn S. A., Schutte M., Hoque A. T., Moskaluk C. A., da Costa L. T., Rozenblum E., Weinstein C. L., Fischer A., Yeo C. J., Hruban R. H. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996 Jan 19;271(5247):350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- Hahn S. A., Seymour A. B., Hoque A. T., Schutte M., da Costa L. T., Redston M. S., Caldas C., Weinstein C. L., Fischer A., Yeo C. J. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995 Oct 15;55(20):4670–4675. [PubMed] [Google Scholar]
- Hruban R. H., van Mansfeld A. D., Offerhaus G. J., van Weering D. H., Allison D. C., Goodman S. N., Kensler T. W., Bose K. K., Cameron J. L., Bos J. L. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993 Aug;143(2):545–554. [PMC free article] [PubMed] [Google Scholar]
- Hruban R. H., van der Riet P., Erozan Y. S., Sidransky D. Brief report: molecular biology and the early detection of carcinoma of the bladder--the case of Hubert H. Humphrey. N Engl J Med. 1994 May 5;330(18):1276–1278. doi: 10.1056/NEJM199405053301805. [DOI] [PubMed] [Google Scholar]
- Matise T. C., Perlin M., Chakravarti A. Automated construction of genetic linkage maps using an expert system (MultiMap): a human genome linkage map. Nat Genet. 1994 Apr;6(4):384–390. doi: 10.1038/ng0494-384. [DOI] [PubMed] [Google Scholar]
- Parker S. L., Tong T., Bolden S., Wingo P. A. Cancer statistics, 1996. CA Cancer J Clin. 1996 Jan-Feb;46(1):5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- Parsa N. Z., Gaidano G., Mukherjee A. B., Hauptschein R. S., Lenoir G., Dalla-Favera R., Chaganti R. S. Cytogenetic and molecular analysis of 6q deletions in Burkitt's lymphoma cell lines. Genes Chromosomes Cancer. 1994 Jan;9(1):13–18. doi: 10.1002/gcc.2870090104. [DOI] [PubMed] [Google Scholar]
- Pellegata N. S., Sessa F., Renault B., Bonato M., Leone B. E., Solcia E., Ranzani G. N. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994 Mar 15;54(6):1556–1560. [PubMed] [Google Scholar]
- Redston M. S., Caldas C., Seymour A. B., Hruban R. H., da Costa L., Yeo C. J., Kern S. E. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1994 Jun 1;54(11):3025–3033. [PubMed] [Google Scholar]
- Schutte M., Hruban R. H., Hedrick L., Cho K. R., Nadasdy G. M., Weinstein C. L., Bova G. S., Isaacs W. B., Cairns P., Nawroz H. DPC4 gene in various tumor types. Cancer Res. 1996 Jun 1;56(11):2527–2530. [PubMed] [Google Scholar]
- Schutte M., da Costa L. T., Hahn S. A., Moskaluk C., Hoque A. T., Rozenblum E., Weinstein C. L., Bittner M., Meltzer P. S., Trent J. M. Identification by representational difference analysis of a homozygous deletion in pancreatic carcinoma that lies within the BRCA2 region. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5950–5954. doi: 10.1073/pnas.92.13.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Omata M., Kawai S., Saisho H., Ohto M., Saiki R. K., Sninsky J. J. Detection of ras gene mutations in pancreatic juice and peripheral blood of patients with pancreatic adenocarcinoma. Cancer Res. 1993 Jun 1;53(11):2472–2474. [PubMed] [Google Scholar]
- Warshaw A. L., Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992 Feb 13;326(7):455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995 Dec 21;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]





