Abstract
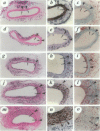
Vascular interventions for atherothrombotic disease frequently induce neointima formation, which can contribute to restenosis of blood vessels. As the molecular mechanisms of this process remain largely unknown, quantitative models of arterial injury in transgenic animals may be useful to study this process at the genetic level. Here, an injury model is proposed in which surgically exposed femoral arteries in mice were injured perivascularly via a single delivery of an electric current. Transmission electron microscopy, light microscopy, and immunohistochemistry revealed that electric injury destroyed all medial smooth muscle cells, denuded the injured segment of intact endothelium, and transiently induced platelet-rich mural thrombosis. A vascular wound-healing response resulted that was characterized by degradation of the mural thrombus, transient infiltration of the vessel wall by inflammatory cells, and progressive removal of the necrotic debris. Topographic analysis revealed repopulation of the media and accumulation in the neointima of smooth muscle cells originating from the uninjured borders and progressing into the necrotic center. Within 3 weeks after injury, a neointima of 0.026 +/- 0.003 mm2 (n = 7 arteries) was formed that contained a maximum of 12 +/- 1 layers of smooth muscle alpha-actin-immunoreactive cells. Evans blue staining in five electrically injured arteries revealed a denuded distance of 2.8 +/- 0.2 mm immediately after injury, which became progressively re-endothelialized from the uninjured borders to 2.2 +/- 0.08 mm (P = 0.013 vs freshly injured by analysis of variance), 0.8 +/- 0.22 mm (P < 0.001), and 0.005 +/- 0.003 mm (P < 0.001) within 2, 7, and 14 days after injury, respectively. Analysis of 5'-bromo-2'-deoxyuridine incorporation revealed that a maximum of 35 +/- 10% endothelial cells proliferated within 2 days after injury and that in the media and neointima, a maximum of, respectively, 12 +/- 2% and 18 +/- 3% smooth muscle cells proliferated within 2 weeks after injury. Thus, electric injury of arteries provides a model of vascular wound healing with arterial neointima formation and re-endothelialization that may be useful for the genetic analysis of its molecular mechanisms in transgenic mice.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz E., Schlote W. Responses of vessel walls to chronically applied electrical stimuli. Basic Res Cardiol. 1979 Jan-Feb;74(1):10–20. doi: 10.1007/BF01907681. [DOI] [PubMed] [Google Scholar]
- Bilder G. E., Kasiewski C. J., Costello R. J., Hodge T. G., Perrone M. H. Electrode cuff-induced changes in DNA and PDGF gene expression in the rat carotid artery. Atherosclerosis. 1993 Apr;100(1):103–112. doi: 10.1016/0021-9150(93)90072-3. [DOI] [PubMed] [Google Scholar]
- Brody W. R., Angeli W. W., Kosek J. C. Histologic fate of the venous coronary artery bypass in dogs. Am J Pathol. 1972 Jan;66(1):111–130. [PMC free article] [PubMed] [Google Scholar]
- Camilleri J. P., Phat V. N., Bruneval P., Tricottet V., Balaton A., Fiessinger J. N., Cormier J. M. Surface healing and histologic maturation of patent polytetrafluoroethylene grafts implanted in patients for up to 60 months. Arch Pathol Lab Med. 1985 Sep;109(9):833–837. [PubMed] [Google Scholar]
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992 Sep;86(3):723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- Cercek B., Sharifi B., Barath P., Bailey L., Forrester J. S. Growth factors in pathogenesis of coronary arterial restenosis. Am J Cardiol. 1991 Nov 4;68(12):24C–33C. doi: 10.1016/0002-9149(91)90220-f. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Reidy M. A. Kinetics of cellular proliferation after arterial injury. III. Endothelial and smooth muscle growth in chronically denuded vessels. Lab Invest. 1986 Mar;54(3):295–303. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A. Prevention of stenosis after vascular reconstruction: pharmacologic control of intimal hyperplasia--a review. J Vasc Surg. 1991 Jun;13(6):885–891. doi: 10.1067/mva.1991.27929. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Schwartz S. M. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985 Jan;56(1):139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- Dilley R. J., McGeachie J. K., Tennant M. The role of cell proliferation and migration in the development of a neo-intimal layer in veins grafted into arteries, in rats. Cell Tissue Res. 1992 Aug;269(2):281–287. doi: 10.1007/BF00319619. [DOI] [PubMed] [Google Scholar]
- Douek P. C., Correa R., Neville R., Unger E. F., Shou M., Banai S., Ferrans V. J., Epstein S. E., Leon M. B., Bonner R. F. Dose-dependent smooth muscle cell proliferation induced by thermal injury with pulsed infrared lasers. Circulation. 1992 Oct;86(4):1249–1256. doi: 10.1161/01.cir.86.4.1249. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Stewart-Lee A. L., Anggård E. E. Arterial response to mechanical injury: balloon catheter de-endothelialization. Atherosclerosis. 1992 Feb;92(2-3):89–104. doi: 10.1016/0021-9150(92)90268-l. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Au Y. P., Clowes A. W., Reidy M. A. Intimal lesion formation in rat carotid arteries after endothelial denudation in absence of medial injury. Arteriosclerosis. 1990 Nov-Dec;10(6):1082–1087. doi: 10.1161/01.atv.10.6.1082. [DOI] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982 Apr;62(2):347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- Glagov S., Ts'ao C. H. Restitution of aortic wall after sustained necrotizing transmural ligation injury. Role of blood cells and artery cells. Am J Pathol. 1975 Apr;79(1):7–30. [PMC free article] [PubMed] [Google Scholar]
- Glagov S., Weisenberg E., Zarins C. K., Stankunavicius R., Kolettis G. J. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987 May 28;316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- Hanke H., Strohschneider T., Oberhoff M., Betz E., Karsch K. R. Time course of smooth muscle cell proliferation in the intima and media of arteries following experimental angioplasty. Circ Res. 1990 Sep;67(3):651–659. doi: 10.1161/01.res.67.3.651. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Chao S., Schwartz S. M., Reidy M. A. Aortic endothelial cell death and replication in normal and lipopolysaccharide-treated rats. Am J Pathol. 1985 Oct;121(1):123–127. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Clowes M. M., Clowes A. W. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ Res. 1988 Oct;63(4):712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Umemura K., Kondoh K., Uematsu T., Nakashima M. Experimental intimal thickening studies using the photochemically induced thrombosis model in the guinea-pig femoral artery. Atherosclerosis. 1994 May;107(1):117–124. doi: 10.1016/0021-9150(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Ip J. H., Fuster V., Israel D., Badimon L., Badimon J., Chesebro J. H. The role of platelets, thrombin and hyperplasia in restenosis after coronary angioplasty. J Am Coll Cardiol. 1991 May;17(6 Suppl B):77B–88B. doi: 10.1016/0735-1097(91)90942-3. [DOI] [PubMed] [Google Scholar]
- Jackson C. L., Raines E. W., Ross R., Reidy M. A. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993 Aug;13(8):1218–1226. doi: 10.1161/01.atv.13.8.1218. [DOI] [PubMed] [Google Scholar]
- Jamal A., Bendeck M., Langille B. L. Structural changes and recovery of function after arterial injury. Arterioscler Thromb. 1992 Mar;12(3):307–317. doi: 10.1161/01.atv.12.3.307. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Hinohara T., Selmon M. R., Braden L. J., Simpson J. B. Primary peripheral arterial stenoses and restenoses excised by transluminal atherectomy: a histopathologic study. J Am Coll Cardiol. 1990 Feb;15(2):419–425. doi: 10.1016/s0735-1097(10)80071-3. [DOI] [PubMed] [Google Scholar]
- Kakuta T., Currier J. W., Haudenschild C. C., Ryan T. J., Faxon D. P. Differences in compensatory vessel enlargement, not intimal formation, account for restenosis after angioplasty in the hypercholesterolemic rabbit model. Circulation. 1994 Jun;89(6):2809–2815. doi: 10.1161/01.cir.89.6.2809. [DOI] [PubMed] [Google Scholar]
- Kocher O., Gabbiani F., Gabbiani G., Reidy M. A., Cokay M. S., Peters H., Hüttner I. Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening. Biochemical and morphologic studies. Lab Invest. 1991 Oct;65(4):459–470. [PubMed] [Google Scholar]
- Kockx M. M., De Meyer G. R., Andries L. J., Bult H., Jacob W. A., Herman A. G. The endothelium during cuff-induced neointima formation in the rabbit carotid artery. Arterioscler Thromb. 1993 Dec;13(12):1874–1884. doi: 10.1161/01.atv.13.12.1874. [DOI] [PubMed] [Google Scholar]
- Kockx M. M., De Meyer G. R., Jacob W. A., Bult H., Herman A. G. Triphasic sequence of neointimal formation in the cuffed carotid artery of the rabbit. Arterioscler Thromb. 1992 Dec;12(12):1447–1457. doi: 10.1161/01.atv.12.12.1447. [DOI] [PubMed] [Google Scholar]
- Lafont A., Guzman L. A., Whitlow P. L., Goormastic M., Cornhill J. F., Chisolm G. M. Restenosis after experimental angioplasty. Intimal, medial, and adventitial changes associated with constrictive remodeling. Circ Res. 1995 Jun;76(6):996–1002. doi: 10.1161/01.res.76.6.996. [DOI] [PubMed] [Google Scholar]
- Lamand M., Lab C., Mignon M., Tressol J. C. A zinc-deficient diet for ruminants: diagnosis and treatment of deficiency. Ann Rech Vet. 1983;14(3):211–215. [PubMed] [Google Scholar]
- Langille B. L., O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986 Jan 24;231(4736):405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Gibbons G. H., Dzau V. J. Cellular and molecular mechanisms of coronary artery restenosis. Coron Artery Dis. 1993 Mar;4(3):254–259. doi: 10.1097/00019501-199303000-00005. [DOI] [PubMed] [Google Scholar]
- Lindner V., Fingerle J., Reidy M. A. Mouse model of arterial injury. Circ Res. 1993 Nov;73(5):792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- Moreno P. R., Falk E., Palacios I. F., Newell J. B., Fuster V., Fallon J. T. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994 Aug;90(2):775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- Muller D. W., Ellis S. G., Topol E. J. Experimental models of coronary artery restenosis. J Am Coll Cardiol. 1992 Feb;19(2):418–432. doi: 10.1016/0735-1097(92)90500-m. [DOI] [PubMed] [Google Scholar]
- Post M. J., Borst C., Kuntz R. E. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. A study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation. 1994 Jun;89(6):2816–2821. doi: 10.1161/01.cir.89.6.2816. [DOI] [PubMed] [Google Scholar]
- Prescott M. F., McBride C. K., Court M. Development of intimal lesions after leukocyte migration into the vascular wall. Am J Pathol. 1989 Nov;135(5):835–846. [PMC free article] [PubMed] [Google Scholar]
- Reidy M. A., Clowes A. W., Schwartz S. M. Endothelial regeneration. V. Inhibition of endothelial regrowth in arteries of rat and rabbit. Lab Invest. 1983 Nov;49(5):569–575. [PubMed] [Google Scholar]
- Reidy M. A. Neointimal proliferation: the role of basic FGF on vascular smooth muscle cell proliferation. Thromb Haemost. 1993 Jul 1;70(1):172–176. [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Richardson M., Hatton M. W., Buchanan M. R., Moore S. Wound healing in the media of the normolipemic rabbit carotid artery injured by air drying or by balloon catheter de-endothelialization. Am J Pathol. 1990 Dec;137(6):1453–1465. [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Asada Y., Marutsuka K., Hatakeyama K., Sumiyoshi A. Tissue factor induces migration of cultured aortic smooth muscle cells. Thromb Haemost. 1996 Mar;75(3):389–392. [PubMed] [Google Scholar]
- Schwartz S. M., Liaw L. Growth control and morphogenesis in the development and pathology of arteries. J Cardiovasc Pharmacol. 1993;21 (Suppl 1):S31–S49. doi: 10.1097/00005344-199321001-00007. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Reidy M. A., O'Brien E. R. Assessment of factors important in atherosclerotic occlusion and restenosis. Thromb Haemost. 1995 Jul;74(1):541–551. [PubMed] [Google Scholar]
- Shi C., Russell M. E., Bianchi C., Newell J. B., Haber E. Murine model of accelerated transplant arteriosclerosis. Circ Res. 1994 Aug;75(2):199–207. doi: 10.1161/01.res.75.2.199. [DOI] [PubMed] [Google Scholar]
- Shi Y., Pieniek M., Fard A., O'Brien J., Mannion J. D., Zalewski A. Adventitial remodeling after coronary arterial injury. Circulation. 1996 Jan 15;93(2):340–348. doi: 10.1161/01.cir.93.2.340. [DOI] [PubMed] [Google Scholar]
- Steele P. M., Chesebro J. H., Stanson A. W., Holmes D. R., Jr, Dewanjee M. K., Badimon L., Fuster V. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ Res. 1985 Jul;57(1):105–112. doi: 10.1161/01.res.57.1.105. [DOI] [PubMed] [Google Scholar]
- Taubman M. B. Tissue factor regulation in vascular smooth muscle: a summary of studies performed using in vivo and in vitro models. Am J Cardiol. 1993 Sep 9;72(8):55C–60C. doi: 10.1016/0002-9149(93)90256-c. [DOI] [PubMed] [Google Scholar]