Abstract
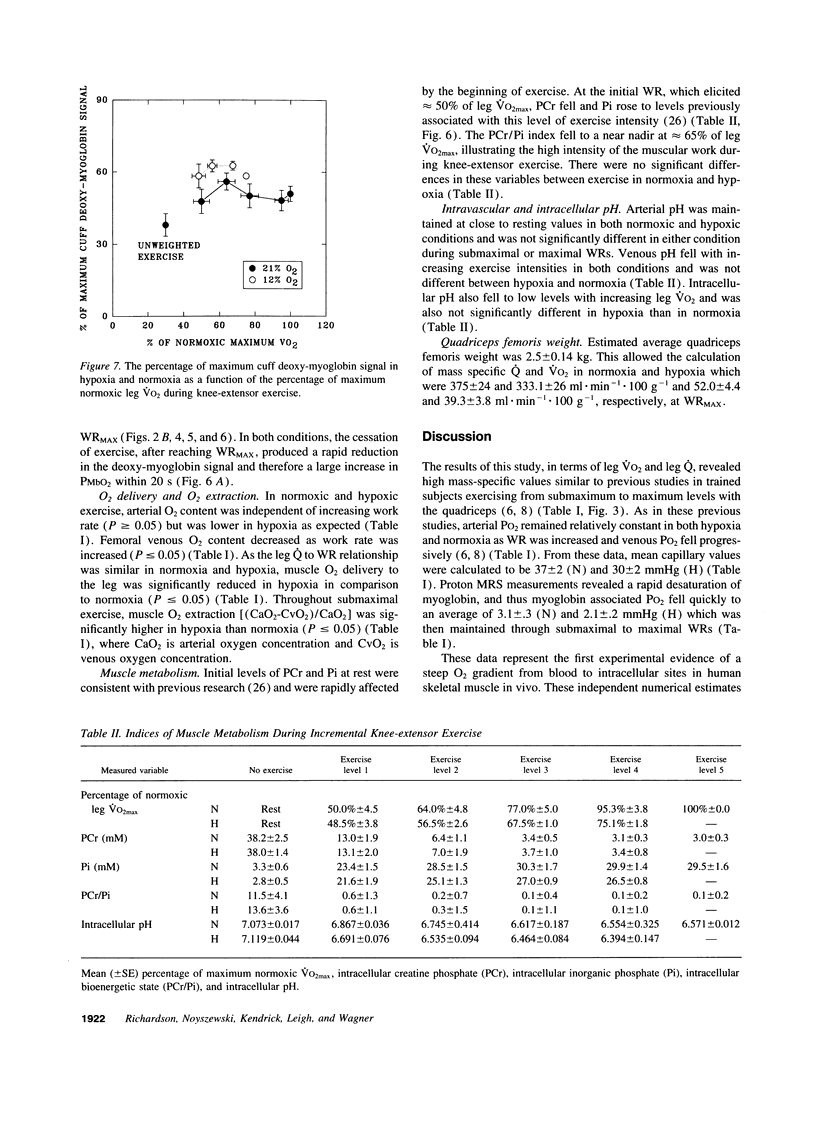
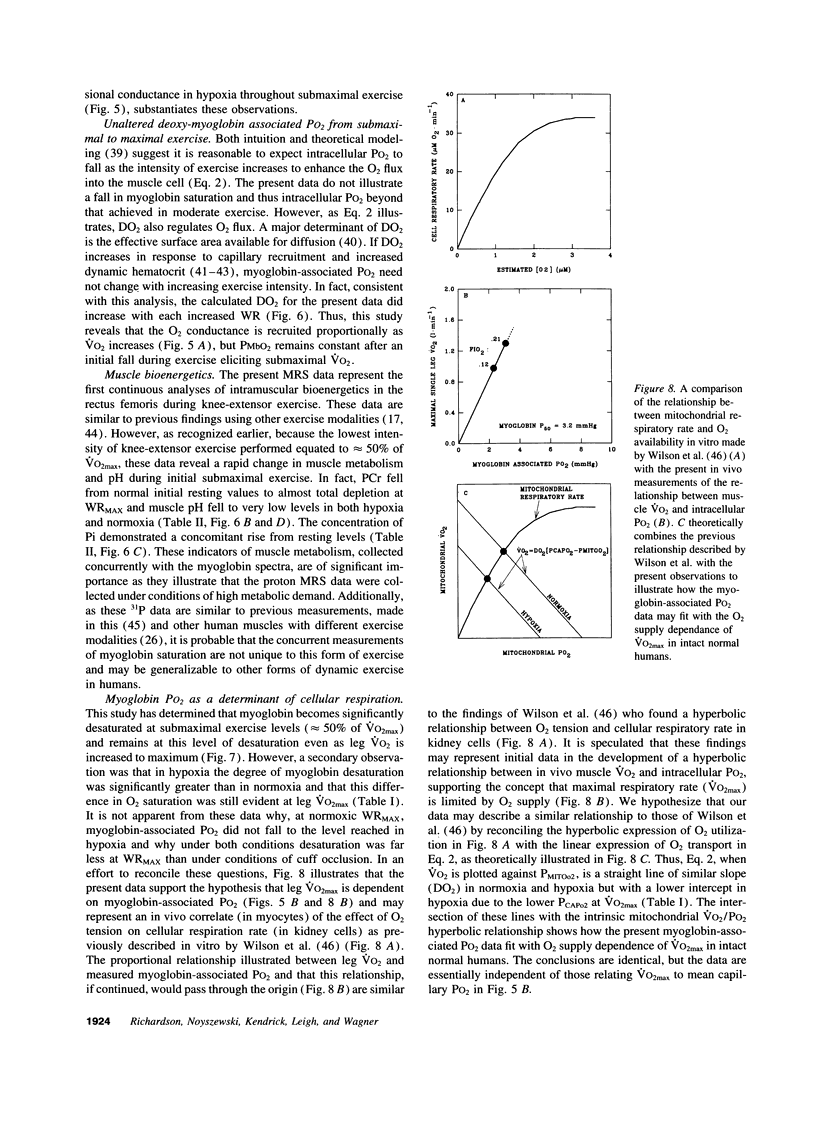
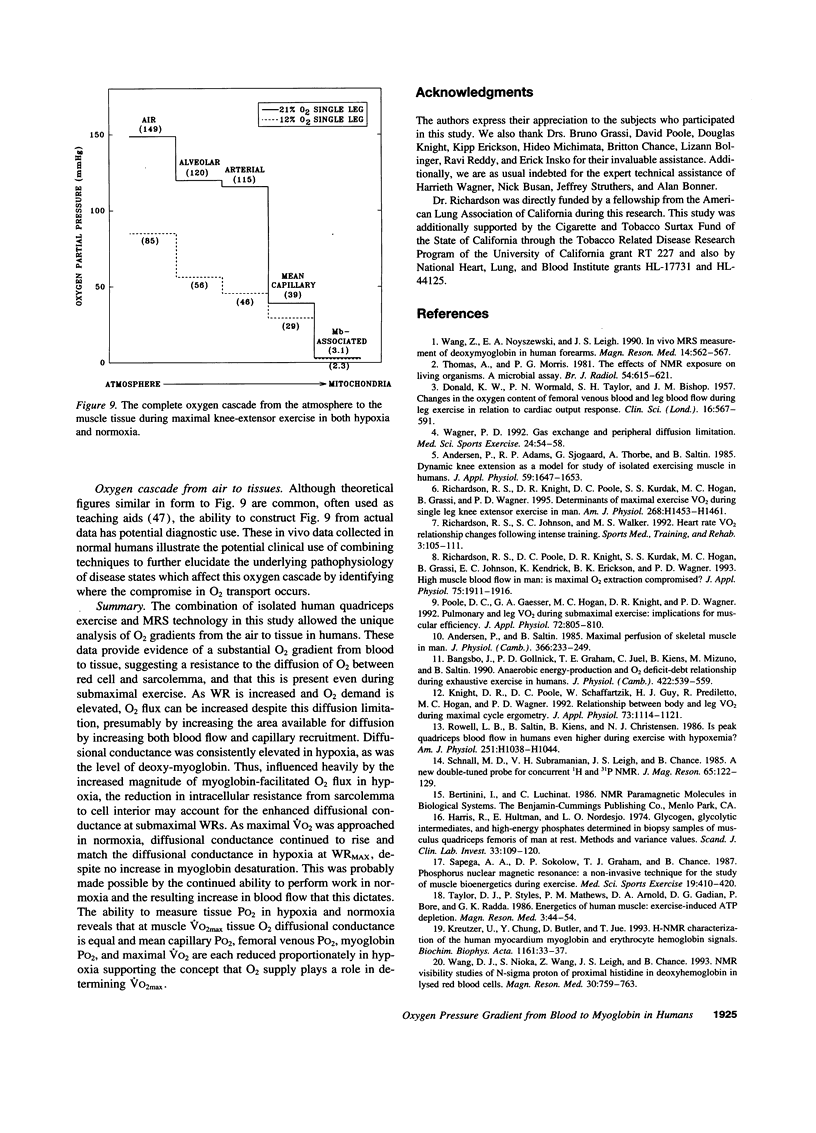
The assumption that cellular oxygen pressure (PO2) is close to zero in maximally exercising muscle is essential for the hypothesis that O2 transport between blood and mitochondria has a finite conductance that determines maximum O2 consumption. The unique combination of isolated human quadriceps exercise, direct measures of arterial, femoral venous PO2, and 1H nuclear magnetic resonance spectroscopy to detect myoglobin desaturation enabled this assumption to be tested in six trained men while breathing room air (normoxic, N) and 12% O2 (hypoxic, H). Within 20 s of exercise onset partial myoglobin desaturation was evident even at 50% of maximum O2 consumption, was significantly greater in H than N, and was then constant at an average of 51 +/- 3% (N) and 60 +/- 3% (H) throughout the incremental exercise protocol to maximum work rate. Assuming a myoglobin PO2 where 50% of myoglobin binding sites are bound with O2 of 3.2 mmHg, myoglobin-associated PO2 averaged 3.1 +/- .3 (N) and 2.1 +/- .2 mmHg (H). At maximal exercise, measurements of arterial PO2 (115 +/- 4 [N] and 46 +/- 1 mmHg [H]) and femoral venous PO2 (22 +/- 1.6 [N] and 17 +/- 1.3 mmHg [H]) resulted in calculated mean capillary PO2 values of 38 +/- 2 (N) and 30 +/- 2 mmHg(H). Thus, for the first time, large differences in PO2 between blood and intracellular tissue have been demonstrated in intact normal human muscle and are found over a wide range of exercise intensities. These data are consistent with an O2 diffusion limitation across the 1-5-microns path-length from red cell to the sarcolemma that plays a role in determining maximal muscle O2 uptake in normal humans.
Full text
PDF

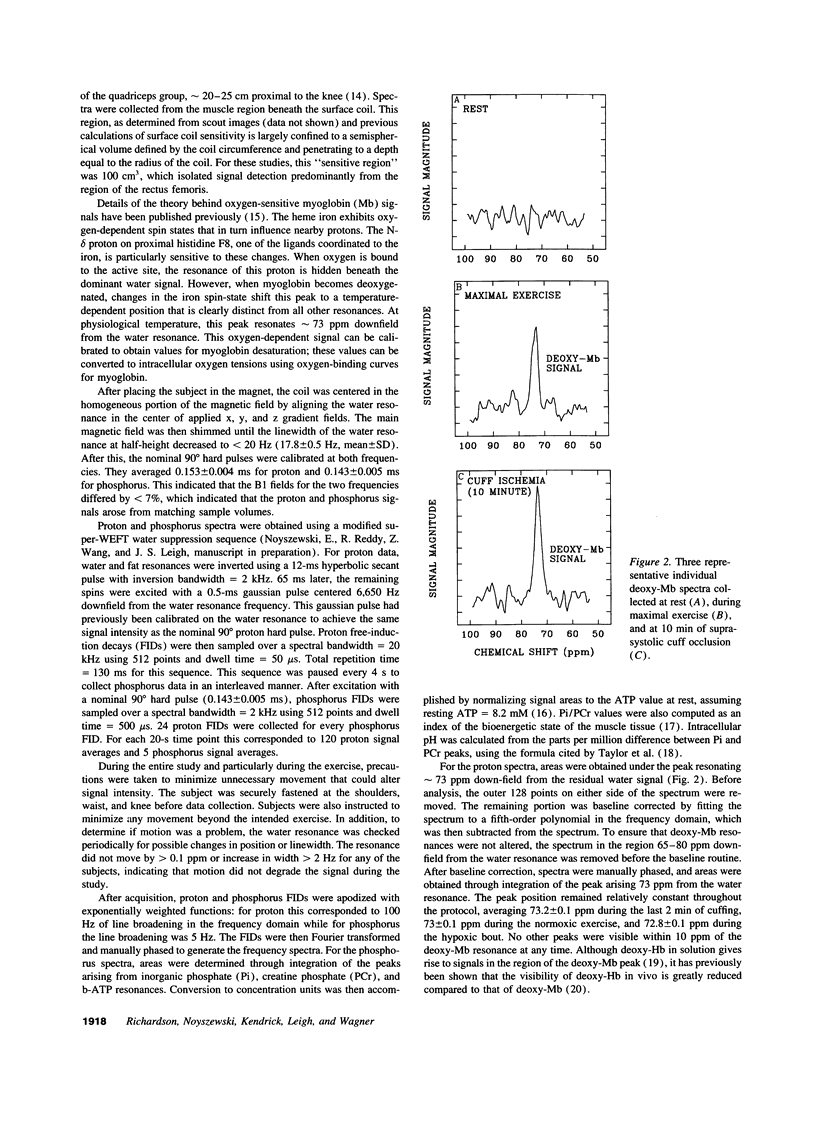
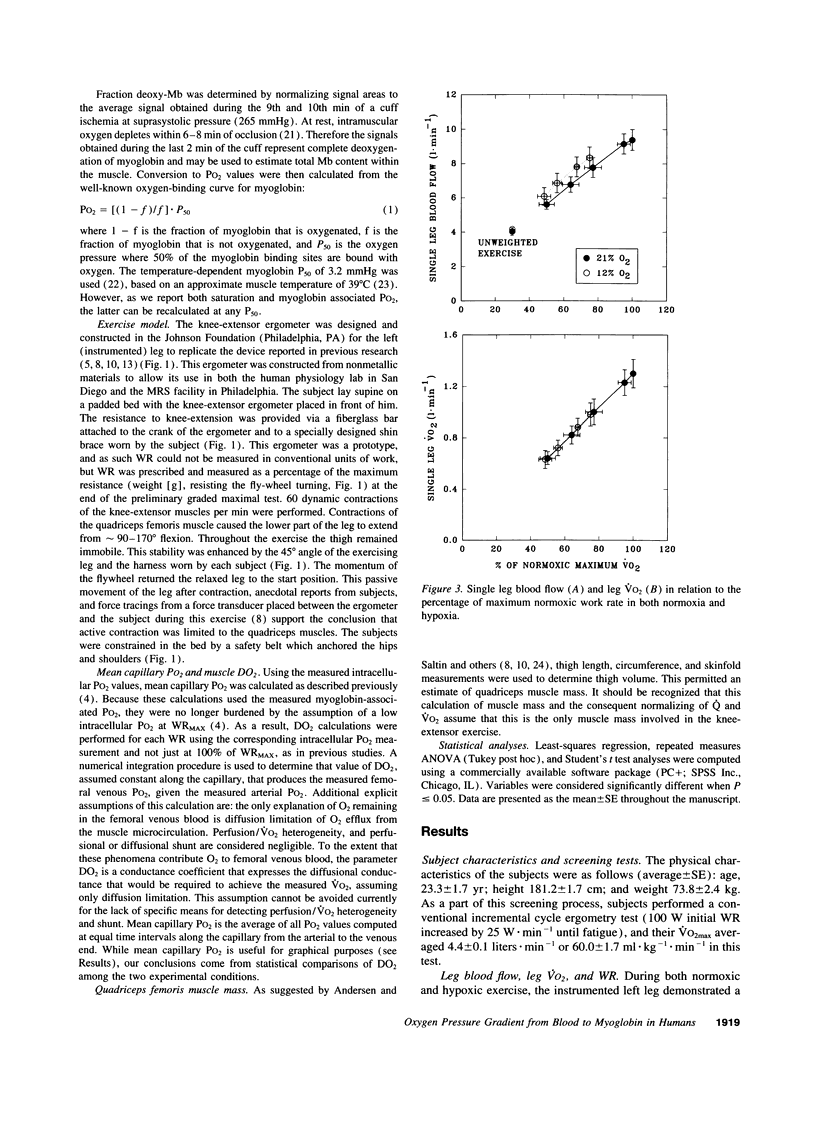
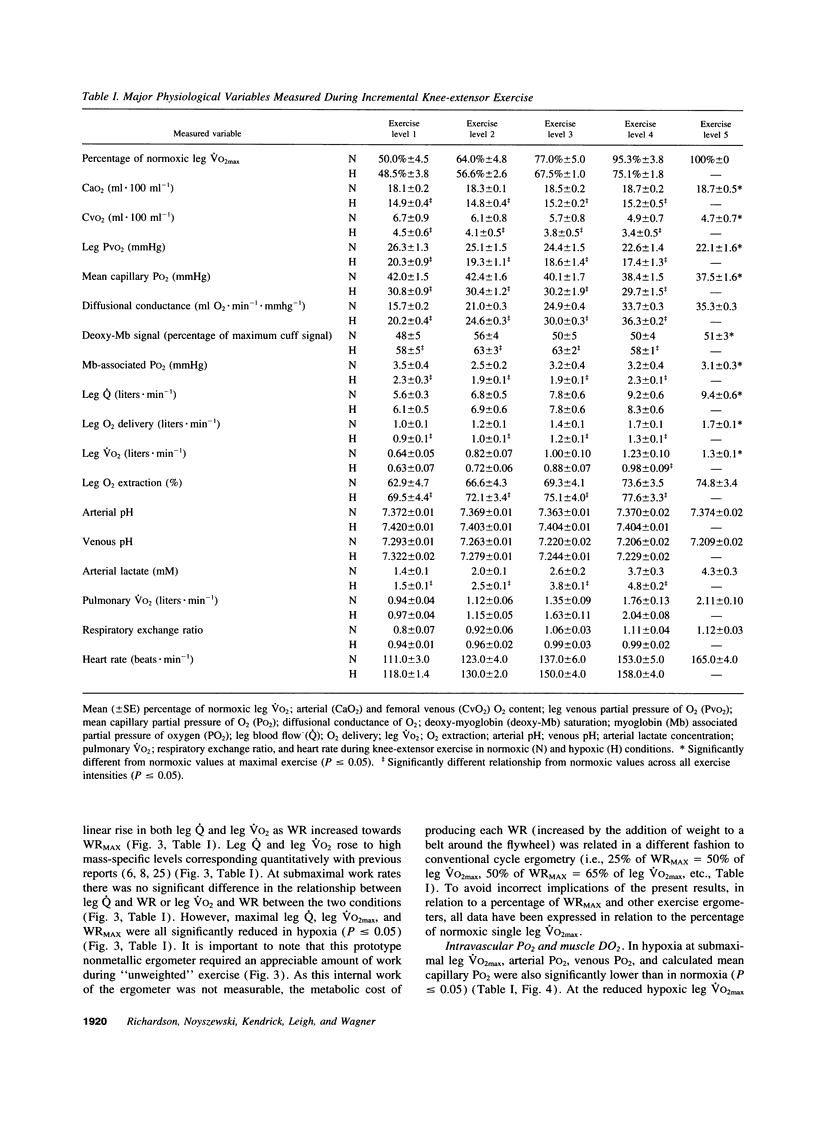
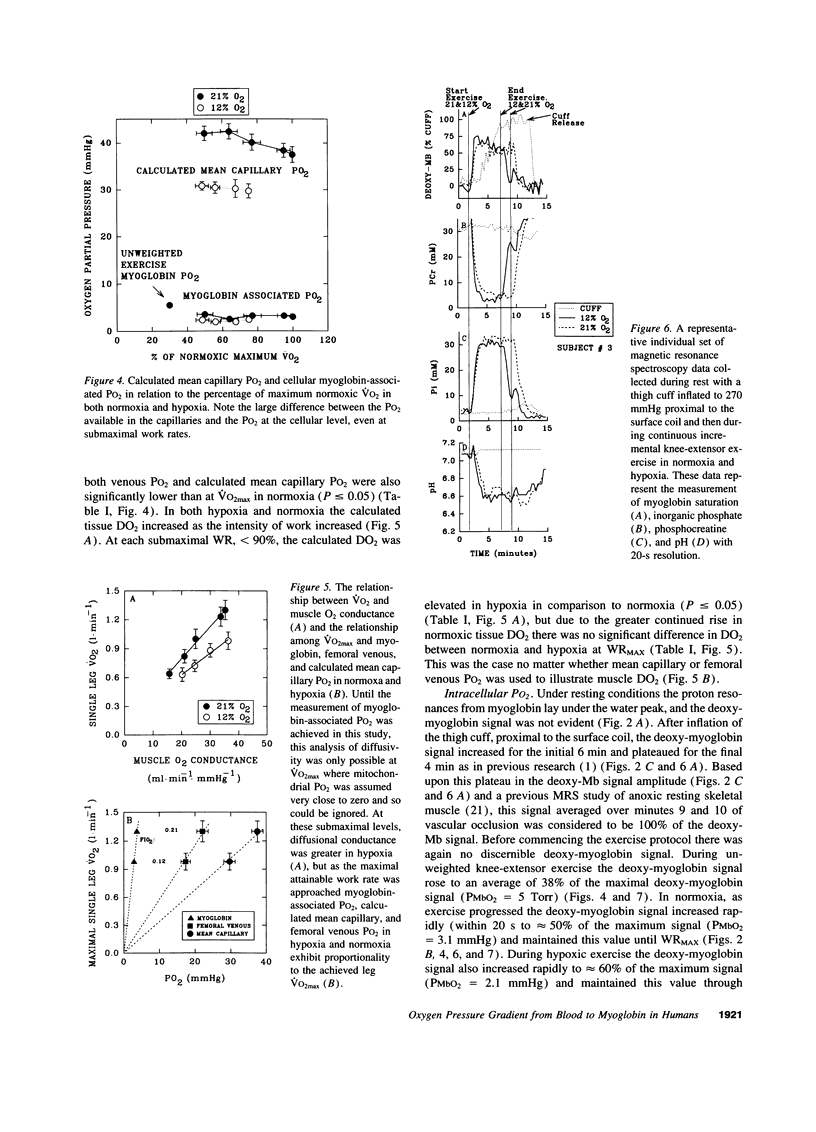


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Adams R. P., Sjøgaard G., Thorboe A., Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 1985 Nov;59(5):1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P., Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985 Sep;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J., Gollnick P. D., Graham T. E., Juel C., Kiens B., Mizuno M., Saltin B. Anaerobic energy production and O2 deficit-debt relationship during exhaustive exercise in humans. J Physiol. 1990 Mar;422:539–559. doi: 10.1113/jphysiol.1990.sp018000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J., Graham T., Johansen L., Saltin B. Muscle lactate metabolism in recovery from intense exhaustive exercise: impact of light exercise. J Appl Physiol (1985) 1994 Oct;77(4):1890–1895. doi: 10.1152/jappl.1994.77.4.1890. [DOI] [PubMed] [Google Scholar]
- Blei M. L., Conley K. E., Kushmerick M. J. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol. 1993 Jun;465:203–222. doi: 10.1113/jphysiol.1993.sp019673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONALD K. W., WORMALD P. N., TAYLOR S. H., BISHOP J. M. Changes in the oxygen content of femoral venous blood and leg blood flow during leg exercise in relation to cardiac output response. Clin Sci. 1957 Aug;16(3):567–591. [PubMed] [Google Scholar]
- Federspiel W. J., Popel A. S. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc Res. 1986 Sep;32(2):164–189. doi: 10.1016/0026-2862(86)90052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayeski T. E., Federspiel W. J., Honig C. R. A graphical analysis of the influence of red cell transit time, carrier-free layer thickness, and intracellular PO2 on blood-tissue O2 transport. Adv Exp Med Biol. 1988;222:25–35. doi: 10.1007/978-1-4615-9510-6_3. [DOI] [PubMed] [Google Scholar]
- Gayeski T. E., Honig C. R. Intracellular PO2 in long axis of individual fibers in working dog gracilis muscle. Am J Physiol. 1988 Jun;254(6 Pt 2):H1179–H1186. doi: 10.1152/ajpheart.1988.254.6.H1179. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. J., Duling B. R. Role of oxygen in arteriolar functional vasodilation in hamster striated muscle. Am J Physiol. 1978 Nov;235(5):H505–H515. doi: 10.1152/ajpheart.1978.235.5.H505. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Nordesjö L. O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974 Apr;33(2):109–120. [PubMed] [Google Scholar]
- Hogan M. C., Richardson R. S., Kurdak S. S. Initial fall in skeletal muscle force development during ischemia is related to oxygen availability. J Appl Physiol (1985) 1994 Nov;77(5):2380–2384. doi: 10.1152/jappl.1994.77.5.2380. [DOI] [PubMed] [Google Scholar]
- Hogan M. C., Roca J., West J. B., Wagner P. D. Dissociation of maximal O2 uptake from O2 delivery in canine gastrocnemius in situ. J Appl Physiol (1985) 1989 Mar;66(3):1219–1226. doi: 10.1152/jappl.1989.66.3.1219. [DOI] [PubMed] [Google Scholar]
- Honig C. R., Gayeski T. E. Resistance to O2 diffusion in anemic red muscle: roles of flux density and cell PO2. Am J Physiol. 1993 Sep;265(3 Pt 2):H868–H875. doi: 10.1152/ajpheart.1993.265.3.H868. [DOI] [PubMed] [Google Scholar]
- Honig C. R., Odoroff C. L., Frierson J. L. Active and passive capillary control in red muscle at rest and in exercise. Am J Physiol. 1982 Aug;243(2):H196–H206. doi: 10.1152/ajpheart.1982.243.2.H196. [DOI] [PubMed] [Google Scholar]
- Hultman E., Bergström J., Anderson N. M. Breakdown and resynthesis of phosphorylcreatine and adenosine triphosphate in connection with muscular work in man. Scand J Clin Lab Invest. 1967;19(1):56–66. doi: 10.3109/00365516709093481. [DOI] [PubMed] [Google Scholar]
- Jones P. R., Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969 Oct;204(2):63P–66P. [PubMed] [Google Scholar]
- Knight D. R., Poole D. C., Schaffartzik W., Guy H. J., Prediletto R., Hogan M. C., Wagner P. D. Relationship between body and leg VO2 during maximal cycle ergometry. J Appl Physiol (1985) 1992 Sep;73(3):1114–1121. doi: 10.1152/jappl.1992.73.3.1114. [DOI] [PubMed] [Google Scholar]
- Knight D. R., Schaffartzik W., Poole D. C., Hogan M. C., Bebout D. E., Wagner P. D. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol (1985) 1993 Dec;75(6):2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- Kreutzer U., Chung Y., Butler D., Jue T. H-NMR characterization of the human myocardium myoglobin and erythrocyte hemoglobin signals. Biochim Biophys Acta. 1993 Jan 15;1161(1):33–37. doi: 10.1016/0167-4838(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Lindbom L., Tuma R. F., Arfors K. E. Influence of oxygen on perfused capillary density and capillary red cell velocity in rabbit skeletal muscle. Microvasc Res. 1980 Mar;19(2):197–208. doi: 10.1016/0026-2862(80)90040-0. [DOI] [PubMed] [Google Scholar]
- Lund N., Damon D. H., Damon D. N., Duling B. R. Capillary grouping in hamster tibials anterior muscles: flow patterns, and physiological significance. Int J Microcirc Clin Exp. 1987;5(4):359–372. [PubMed] [Google Scholar]
- Piiper J., Haab P. Oxygen supply and uptake in tissue models with unequal distribution of blood flow and shunt. Respir Physiol. 1991 May;84(2):261–271. doi: 10.1016/0034-5687(91)90122-y. [DOI] [PubMed] [Google Scholar]
- Piiper J. Unequal distribution of blood flow in exercising muscle of the dog. Respir Physiol. 1990 May-Jun;80(2-3):129–136. doi: 10.1016/0034-5687(90)90076-b. [DOI] [PubMed] [Google Scholar]
- Poole D. C., Gaesser G. A., Hogan M. C., Knight D. R., Wagner P. D. Pulmonary and leg VO2 during submaximal exercise: implications for muscular efficiency. J Appl Physiol (1985) 1992 Feb;72(2):805–810. doi: 10.1152/jappl.1992.72.2.805. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E. Studies on the oxygen and carbon monoxide equilibria of human myoglobin. Arch Biochem Biophys. 1958 Oct;77(2):478–492. doi: 10.1016/0003-9861(58)90094-8. [DOI] [PubMed] [Google Scholar]
- Richardson R. S., Knight D. R., Poole D. C., Kurdak S. S., Hogan M. C., Grassi B., Wagner P. D. Determinants of maximal exercise VO2 during single leg knee-extensor exercise in humans. Am J Physiol. 1995 Apr;268(4 Pt 2):H1453–H1461. doi: 10.1152/ajpheart.1995.268.4.H1453. [DOI] [PubMed] [Google Scholar]
- Richardson R. S., Poole D. C., Knight D. R., Kurdak S. S., Hogan M. C., Grassi B., Johnson E. C., Kendrick K. F., Erickson B. K., Wagner P. D. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol (1985) 1993 Oct;75(4):1911–1916. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- Rowell L. B., Saltin B., Kiens B., Christensen N. J. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol. 1986 Nov;251(5 Pt 2):H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- Saltin B., Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol. 1966 Nov;21(6):1757–1762. doi: 10.1152/jappl.1966.21.6.1757. [DOI] [PubMed] [Google Scholar]
- Sapega A. A., Sokolow D. P., Graham T. J., Chance B. Phosphorus nuclear magnetic resonance: a non-invasive technique for the study of muscle bioenergetics during exercise. Med Sci Sports Exerc. 1987 Aug;19(4):410–420. [PubMed] [Google Scholar]
- Severinghaus J. W. Exercise O2 transport model assuming zero cytochrome PO2 at VO2 max. J Appl Physiol (1985) 1994 Aug;77(2):671–678. doi: 10.1152/jappl.1994.77.2.671. [DOI] [PubMed] [Google Scholar]
- Systrom D. M., Kanarek D. J., Kohler S. J., Kazemi H. 31P nuclear magnetic resonance spectroscopy study of the anaerobic threshold in humans. J Appl Physiol (1985) 1990 May;68(5):2060–2066. doi: 10.1152/jappl.1990.68.5.2060. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Styles P., Matthews P. M., Arnold D. A., Gadian D. G., Bore P., Radda G. K. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med. 1986 Feb;3(1):44–54. doi: 10.1002/mrm.1910030107. [DOI] [PubMed] [Google Scholar]
- Thomas A., Morris P. G. The effects of NMR exposure on living organisms. I. A microbial assay. Br J Radiol. 1981 Jul;54(643):615–621. doi: 10.1259/0007-1285-54-643-615. [DOI] [PubMed] [Google Scholar]
- Wagner P. D. Gas exchange and peripheral diffusion limitation. Med Sci Sports Exerc. 1992 Jan;24(1):54–58. [PubMed] [Google Scholar]
- Wang D. J., Nioka S., Wang Z., Leigh J. S., Chance B. NMR visibility studies of N-delta proton of proximal histidine in deoxyhemoglobin in lysed and intact red cells. Magn Reson Med. 1993 Dec;30(6):759–763. doi: 10.1002/mrm.1910300616. [DOI] [PubMed] [Google Scholar]
- Wang Z. Y., Noyszewski E. A., Leigh J. S., Jr In vivo MRS measurement of deoxymyoglobin in human forearms. Magn Reson Med. 1990 Jun;14(3):562–567. doi: 10.1002/mrm.1910140314. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Erecińska M., Drown C., Silver I. A. Effect of oxygen tension on cellular energetics. Am J Physiol. 1977 Nov;233(5):C135–C140. doi: 10.1152/ajpcell.1977.233.5.C135. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B. Transport of oxygen in muscle. Annu Rev Physiol. 1989;51:857–878. doi: 10.1146/annurev.ph.51.030189.004233. [DOI] [PubMed] [Google Scholar]



