Abstract
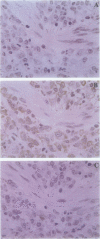
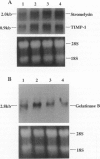
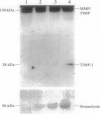
Scleritis is a sight-threatening inflammatory disorder of the eye characterized by the degradation of scleral matrix. Matrix metalloproteinases (MMPs) are ubiquitous proteolytic enzymes important in physiological and pathological processes, the activity of which is stringently controlled by the action of a family of natural antagonists, the tissue inhibitors of matrix metalloproteinases (TIMPs). We hypothesized that enhanced expression of MMPs, without the negative regulatory influence of TIMPs, may be a key feature of tissue destruction in inflammatory eye diseases, such as scleritis. The aim of this study was to localize and characterize cells expressing MMPs and TIMPs in sclera affected by necrotizing scleritis and, in a parallel study, to establish whether cytokines modulate MMP expression in cultured human scleral fibroblasts. In situ hybridization and immunohistochemical analyses indicated that resident scleral fibroblasts as well as inflammatory cells such as macrophages and T lymphocytes express stromelysin, gelatinase B, and TIMP-1 in necrotizing scleritis tissue. In addition, cytoplasmic immunoreactivity for tumor necrosis factor-alpha, an inducer of MMPs, was detected in infiltrating inflammatory cells. Cultured scleral fibroblasts stimulated with the combination of interleukin-1 alpha plus tumor necrosis factor-alpha increased TIMP-1 mRNA twofold above constitutive levels. By contrast, these cytokines induced a sevenfold increase in the steady-state levels of stromelysin mRNA. Using Western blotting, stromelysin and TIMP-1 protein production paralleled mRNA induction in cytokine-stimulated human scleral fibroblasts. Culture supernatants harvested from cytokine-stimulated human scleral fibroblasts were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis gelatin substrate zymography. Our results revealed a prominent 92-kd gelatinolytic band corresponding to gelatinase B, which was inducible with interleukin-1 alpha. These data provide evidence for our hypothesis, that an imbalance between enzyme/inhibitor ratios may be the underlying mechanism of the tissue destruction characteristic of scleritis. Our results demonstrate the potential involvement of MMPs and their modulation by cytokines produced by infiltrating inflammatory cells in destructive ocular inflammation.
Full text
PDF




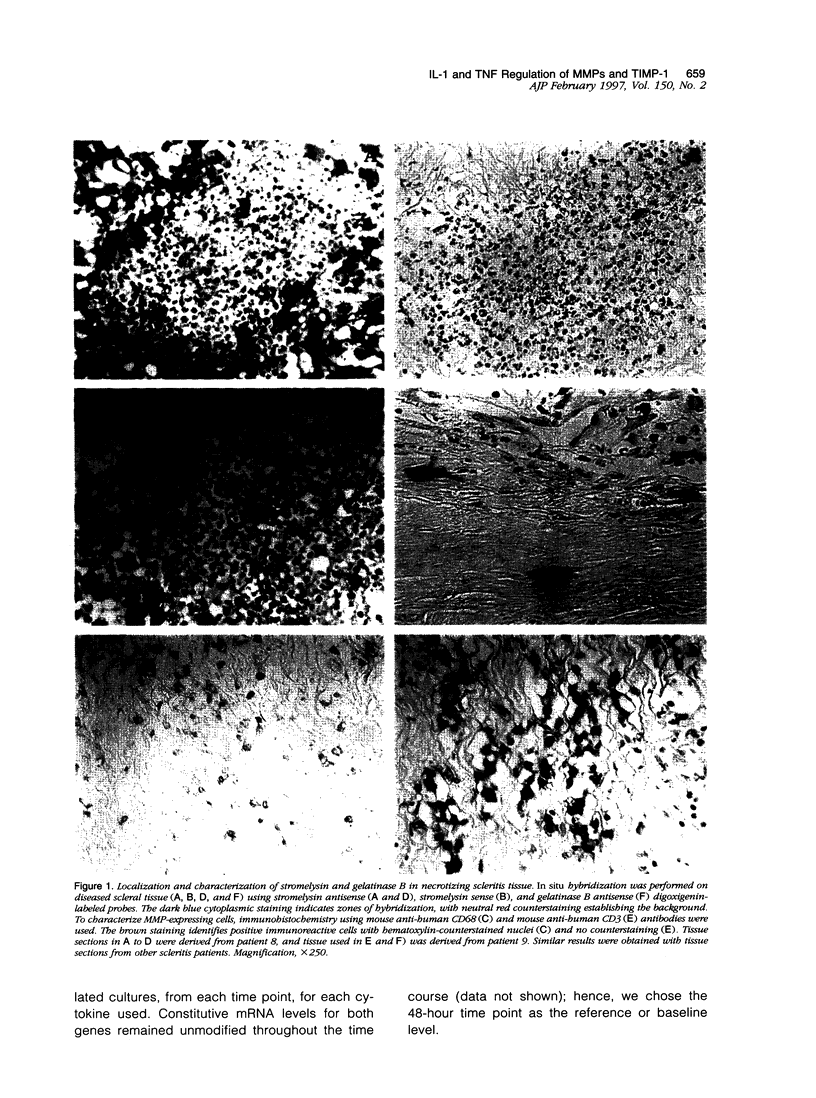
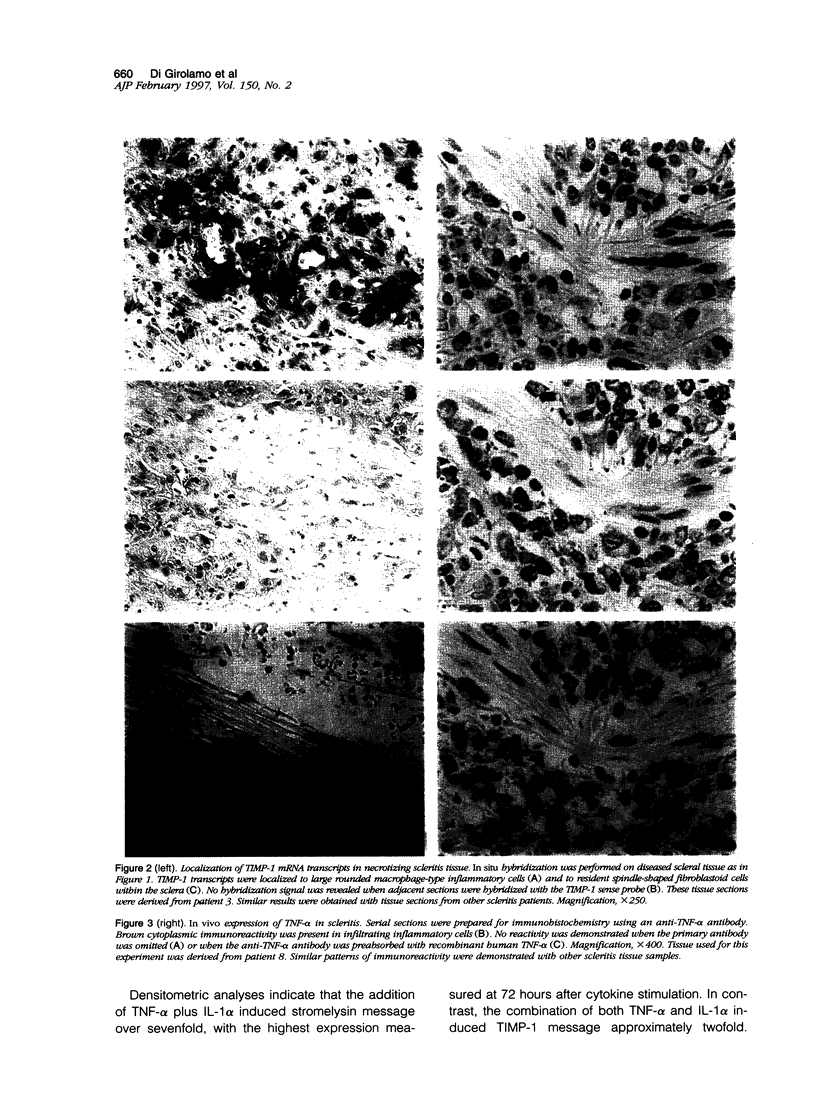
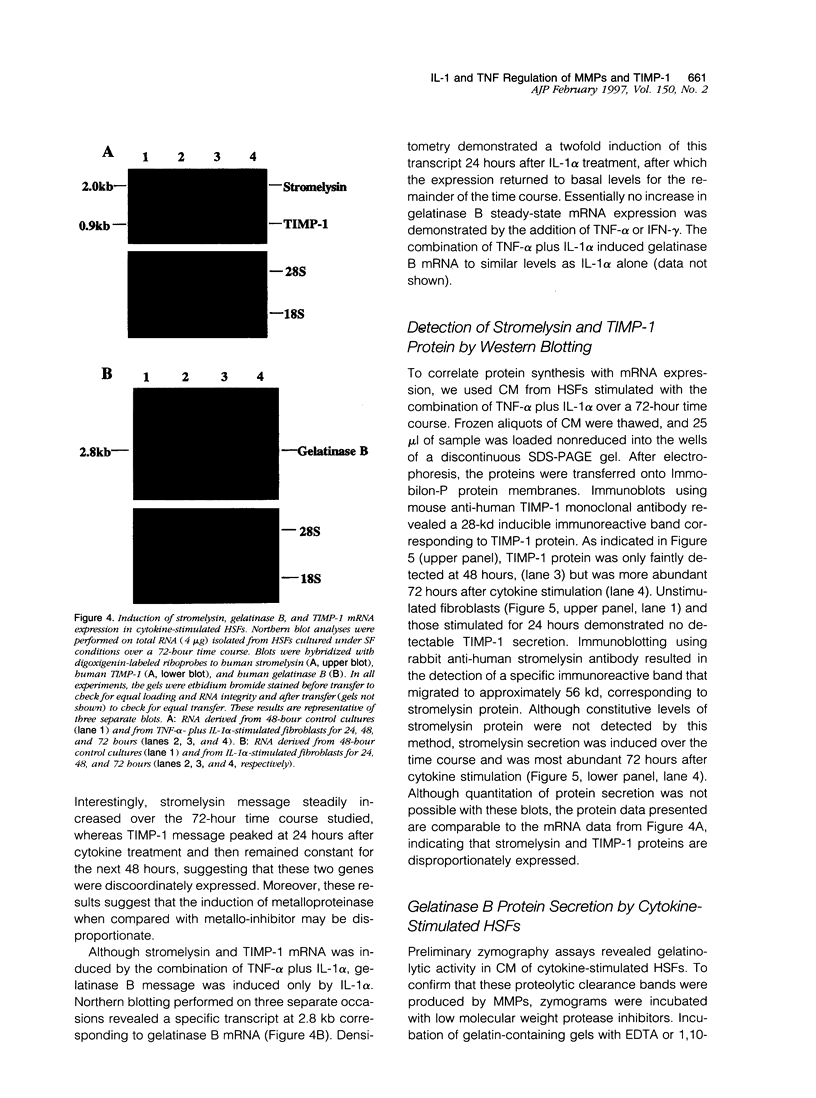
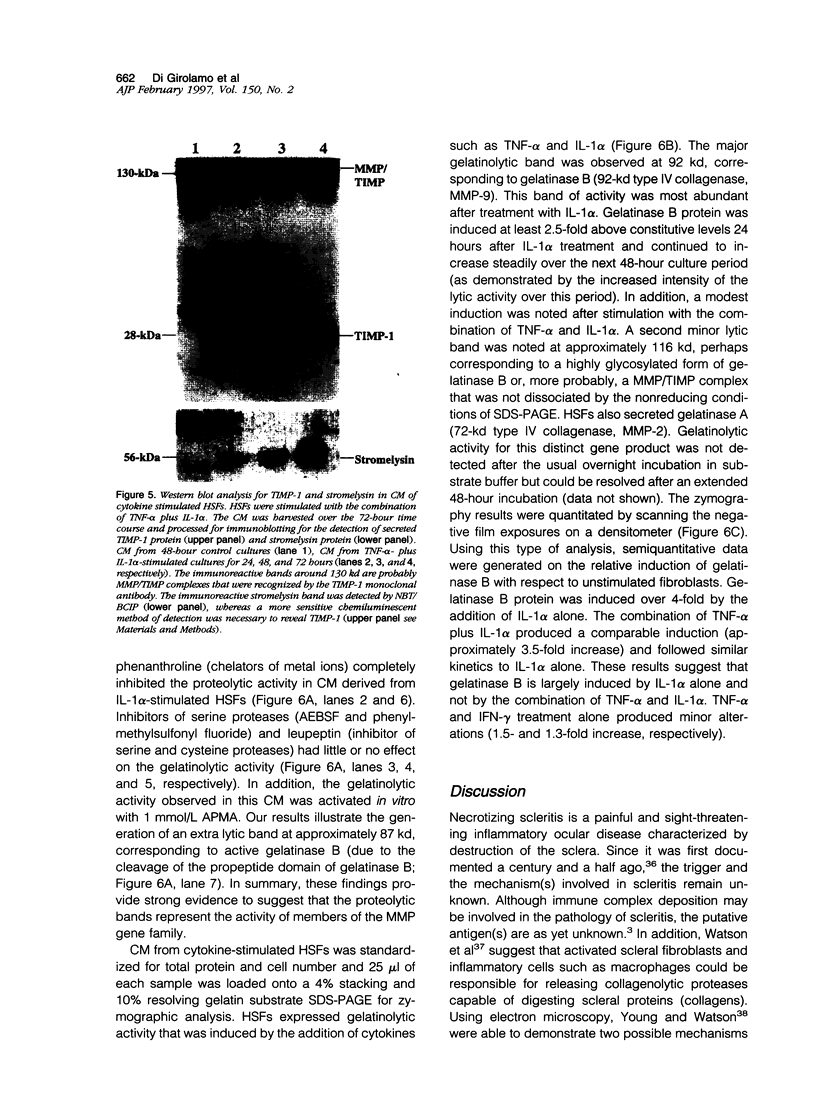



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal C., Hembree J. R., Rorke E. A., Eckert R. L. Transforming growth factor beta 1 regulation of metalloproteinase production in cultured human cervical epithelial cells. Cancer Res. 1994 Feb 15;54(4):943–949. [PubMed] [Google Scholar]
- Ahmed A. A., Nordlind K., Schultzberg M., Lidén S. Interleukin-1 alpha- and beta-, interleukin-6- and tumour necrosis factor-alpha-like immunoreactivities in chronic granulomatous skin conditions. Acta Derm Venereol. 1994 Nov;74(6):435–440. doi: 10.2340/0001555574435440. [DOI] [PubMed] [Google Scholar]
- Aimes R. T., Quigley J. P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995 Mar 17;270(11):5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conca W., Kaplan P. B., Krane S. M. Increases in levels of procollagenase messenger RNA in cultured fibroblasts induced by human recombinant interleukin 1 beta or serum follow c-jun expression and are dependent on new protein synthesis. J Clin Invest. 1989 May;83(5):1753–1757. doi: 10.1172/JCI114077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo N., Underwood A., McCluskey P. J., Wakefield D. Functional activity of plasma fibronectin in patients with diabetes mellitus. Diabetes. 1993 Nov;42(11):1606–1613. doi: 10.2337/diab.42.11.1606. [DOI] [PubMed] [Google Scholar]
- Evans P. J., Eustace P. Scleromalacia perforans associated with Crohn's disease. Treated with sodium versenate (EDTA). Br J Ophthalmol. 1973 May;57(5):330–335. doi: 10.1136/bjo.57.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Wu J. J. Collagen structure and cartilage matrix integrity. J Rheumatol Suppl. 1995 Feb;43:82–85. [PubMed] [Google Scholar]
- Firestein G. S. Mechanisms of tissue destruction and cellular activation in rheumatoid arthritis. Curr Opin Rheumatol. 1992 Jun;4(3):348–354. doi: 10.1097/00002281-199206000-00012. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M. Stromelysin and tissue inhibitor of metalloproteinases gene expression in rheumatoid arthritis synovium. Am J Pathol. 1992 Jun;140(6):1309–1314. [PMC free article] [PubMed] [Google Scholar]
- Gress T. M., Müller-Pillasch F., Lerch M. M., Friess H., Büchler M., Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int J Cancer. 1995 Aug 9;62(4):407–413. doi: 10.1002/ijc.2910620409. [DOI] [PubMed] [Google Scholar]
- Herron G. S., Banda M. J., Clark E. J., Gavrilovic J., Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986 Feb 25;261(6):2814–2818. [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hunt R. C., Fox A., al Pakalnis V., Sigel M. M., Kosnosky W., Choudhury P., Black E. P. Cytokines cause cultured retinal pigment epithelial cells to secrete metalloproteinases and to contract collagen gels. Invest Ophthalmol Vis Sci. 1993 Oct;34(11):3179–3186. [PubMed] [Google Scholar]
- Khokha R., Denhardt D. T. Matrix metalloproteinases and tissue inhibitor of metalloproteinases: a review of their role in tumorigenesis and tissue invasion. Invasion Metastasis. 1989;9(6):391–405. [PubMed] [Google Scholar]
- Lelièvre Y., Bouboutou R., Boiziau J., Faucher D., Achard D., Cartwright T. Low molecular weight, sequence based, collagenase inhibitors selectively block the interaction between collagenase and TIMP (tissue inhibitor of metalloproteinases). Matrix. 1990 Oct;10(5):292–299. doi: 10.1016/s0934-8832(11)80184-8. [DOI] [PubMed] [Google Scholar]
- Leppert D., Waubant E., Galardy R., Bunnett N. W., Hauser S. L. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol. 1995 May 1;154(9):4379–4389. [PubMed] [Google Scholar]
- Lohmander L. S., Hoerrner L. A., Lark M. W. Metalloproteinases, tissue inhibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum. 1993 Feb;36(2):181–189. doi: 10.1002/art.1780360207. [DOI] [PubMed] [Google Scholar]
- MacNaul K. L., Chartrain N., Lark M., Tocci M. J., Hutchinson N. I. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases-1 in rheumatoid human synovial fibroblasts. Synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990 Oct 5;265(28):17238–17245. [PubMed] [Google Scholar]
- Martel-Pelletier J., McCollum R., Fujimoto N., Obata K., Cloutier J. M., Pelletier J. P. Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest. 1994 Jun;70(6):807–815. [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Mauviel A. Cytokine regulation of metalloproteinase gene expression. J Cell Biochem. 1993 Dec;53(4):288–295. doi: 10.1002/jcb.240530404. [DOI] [PubMed] [Google Scholar]
- McDonnell S., Matrisian L. M. Stromelysin in tumor progression and metastasis. Cancer Metastasis Rev. 1990 Dec;9(4):305–319. doi: 10.1007/BF00049521. [DOI] [PubMed] [Google Scholar]
- Mitchell P. G., Magna H. A., Reeves L. M., Lopresti-Morrow L. L., Yocum S. A., Rosner P. J., Geoghegan K. F., Hambor J. E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996 Feb 1;97(3):761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q., Mort J. S., Roughley P. J. Preferential mRNA expression of prostromelysin relative to procollagenase and in situ localization in human articular cartilage. J Clin Invest. 1992 Apr;89(4):1189–1197. doi: 10.1172/JCI115702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata Y., Enghild J. J., Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992 Feb 25;267(6):3581–3584. [PubMed] [Google Scholar]
- Quinones S., Saus J., Otani Y., Harris E. D., Jr, Kurkinen M. Transcriptional regulation of human stromelysin. J Biol Chem. 1989 May 15;264(14):8339–8344. [PubMed] [Google Scholar]
- Rada J. A., Brenza H. L. Increased latent gelatinase activity in the sclera of visually deprived chicks. Invest Ophthalmol Vis Sci. 1995 Jul;36(8):1555–1565. [PubMed] [Google Scholar]
- Rao N. A., Marak G. E., Hidayat A. A. Necrotizing scleritis. A clinico-pathologic study of 41 cases. Ophthalmology. 1985 Nov;92(11):1542–1549. [PubMed] [Google Scholar]
- Ray J. M., Stetler-Stevenson W. G. The role of matrix metalloproteases and their inhibitors in tumour invasion, metastasis and angiogenesis. Eur Respir J. 1994 Nov;7(11):2062–2072. [PubMed] [Google Scholar]
- Ruco L. P., Stoppacciaro A., Pomponi D., Boraschi D., Santoni A., Tagliabue A., Uccini S., Baroni C. D. Immunoreactivity for IL-1 beta and TNF alpha in human lymphoid and nonlymphoid tissues. Am J Pathol. 1989 Nov;135(5):889–897. [PMC free article] [PubMed] [Google Scholar]
- Sainz de la Maza M., Foster C. S., Jabbur N. S. Scleritis associated with systemic vasculitic diseases. Ophthalmology. 1995 Apr;102(4):687–692. doi: 10.1016/s0161-6420(95)30970-0. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Welgus H. G. Human skin fibroblast collagenase inhibitor. Purification and biochemical characterization. J Biol Chem. 1983 Oct 25;258(20):12252–12258. [PubMed] [Google Scholar]
- Takino T., Sato H., Shinagawa A., Seiki M. Identification of the second membrane-type matrix metalloproteinase (MT-MMP-2) gene from a human placenta cDNA library. MT-MMPs form a unique membrane-type subclass in the MMP family. J Biol Chem. 1995 Sep 29;270(39):23013–23020. doi: 10.1074/jbc.270.39.23013. [DOI] [PubMed] [Google Scholar]
- Tuft S. J., Watson P. G. Progression of scleral disease. Ophthalmology. 1991 Apr;98(4):467–471. doi: 10.1016/s0161-6420(91)32269-3. [DOI] [PubMed] [Google Scholar]
- Watson P. G. Doyne Memorial Lecture, 1982. The nature and the treatment of scleral inflammation. Trans Ophthalmol Soc U K. 1982 Jul;102(Pt 2):257–281. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Young R. D., Watson P. G. Microscopical studies of necrotising scleritis. II. Collagen degradation in the scleral stroma. Br J Ophthalmol. 1984 Nov;68(11):781–789. doi: 10.1136/bjo.68.11.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Bernhard E. J., Fox F. E., Billings P. C. Induction of metalloproteinase activity in human T-lymphocytes. Biochim Biophys Acta. 1993 Jun 6;1177(2):174–178. doi: 10.1016/0167-4889(93)90037-p. [DOI] [PubMed] [Google Scholar]
- Zucker S., Conner C., DiMassmo B. I., Ende H., Drews M., Seiki M., Bahou W. F. Thrombin induces the activation of progelatinase A in vascular endothelial cells. Physiologic regulation of angiogenesis. J Biol Chem. 1995 Oct 6;270(40):23730–23738. doi: 10.1074/jbc.270.40.23730. [DOI] [PubMed] [Google Scholar]