Abstract
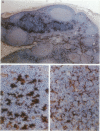
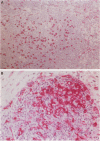
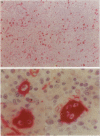
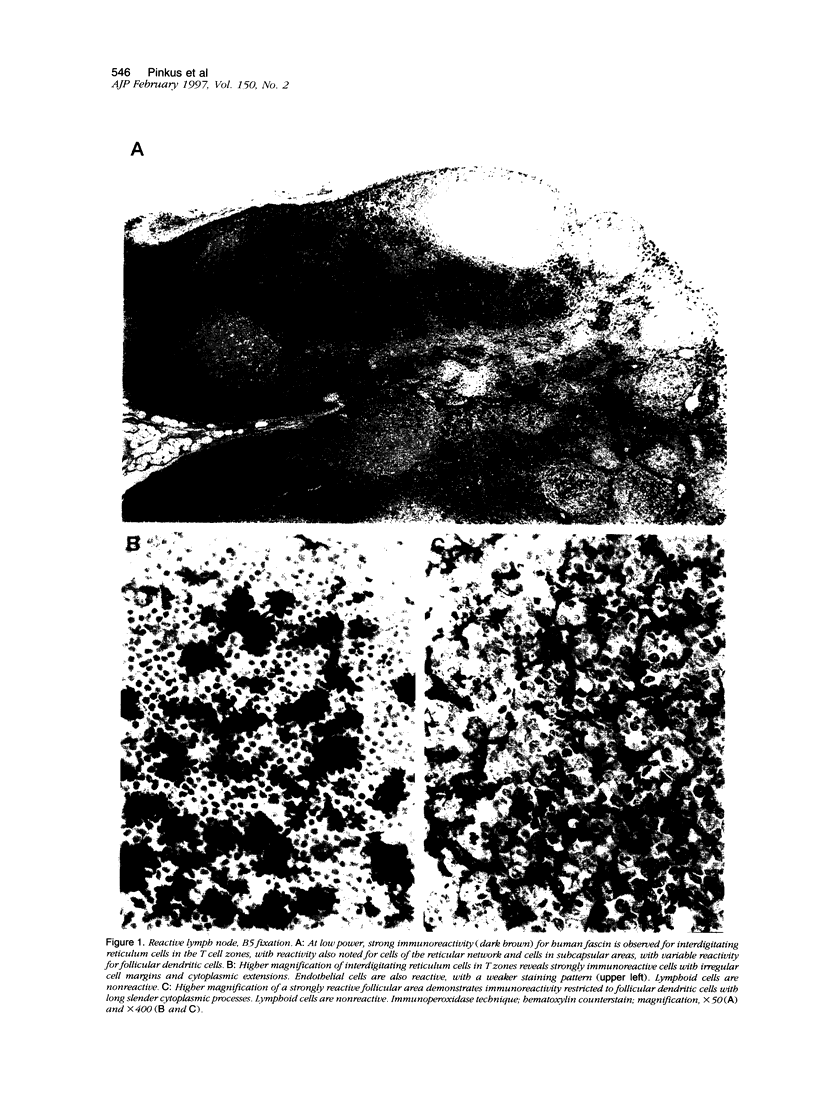
Immunohistochemical localization of human fascin, a distinct 55-kd actin-bundling protein, was determined for a wide variety of lymphoid tissues (364 specimens total). In non-neoplastic tissues, reactivity was highly selective and localized predominantly in dendritic cells. In the thymus, this protein was distinctly localized to medullary dendritic cells. In reactive nodes, interdigitating reticulum cells of T zones, cells in subcapsular areas, and cells of the reticular network were reactive, with variable reactivity observed for follicular dendritic cells. Splenic dendritic cells of the white pulp and sinus-lining cells of the red pulp were reactive. Endothelial cells of all tissues exhibited variable reactivity. Lymphoid cells, myeloid cells, and plasma cells were uniformly nonreactive. In the peripheral blood, only dendritic (veiled) cells were reactive for fascin. A striking finding was observed for cases of Hodgkin's disease (total 187 cases). In all cases of nodular sclerosis (132), mixed cellularity (34), lymphocyte depletion (2), and unclassified types (5), all or nearly all Reed-Sternberg cells and variants were immunoreactive for fascin. Neoplastic cells exhibited strong diffuse cytoplasmic staining and frequently assumed dendritic shapes, particularly in the nodular sclerosis type, producing an interdigitating meshwork or syncytial network of cells. In cases of mixed cellularity type, neoplastic cells generally appeared more discrete. In all 14 cases of nodular lymphocyte predominance type, L&H variants were nonreactive. By contrast, neoplastic lymphoid cells of only 24 of 156 (15%) other lymphoid neoplasms (127 B cell, 27 T cell, and two null cell evaluated) were reactive for fascin. Fascin represents a highly effective marker for detection of certain dendritic cells in normal and neoplastic tissues, is an extremely consistent marker for Reed-Sternberg cells and variants of Hodgkin's disease (except L&H types), and may be helpful to distinguish between Hodgkin's disease and non-Hodgkin's lymphoma in difficult cases. The staining profile for fascin raises the possibility of a dendritic cell derivation, particularly an interdigitating reticulum cell, for the neoplastic cells of Hodgkin's disease, notably in nodular sclerosis type. However, as fascin expression may be induced by Epstein-Barr virus infection of B cells, the possibility that viral induction of fascin in lymphoid or other cell types must also be considered in Epstein-Barr virus-positive cases.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnarsson B. A., Kadin M. E. The immunophenotype of Reed-Sternberg cells. A study of 50 cases of Hodgkin's disease using fixed frozen tissues. Cancer. 1989 Jun 1;63(11):2083–2087. doi: 10.1002/1097-0142(19890601)63:11<2083::aid-cncr2820631102>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Aisenberg A. C. Manifestations of immunologic unresponsiveness in Hodgkin's disease. Cancer Res. 1966 Jun;26(6):1152–1164. [PubMed] [Google Scholar]
- Athan E., Chadburn A., Knowles D. M. The bcl-2 gene translocation is undetectable in Hodgkin's disease by Southern blot hybridization and polymerase chain reaction. Am J Pathol. 1992 Jul;141(1):193–201. [PMC free article] [PubMed] [Google Scholar]
- Beckstead J. H., Warnke R., Bainton D. F. Histochemistry of Hodgkin's disease. Cancer Treat Rep. 1982 Apr;66(4):609–613. [PubMed] [Google Scholar]
- Biniaminov M., Ramot B. Letter: Possible T-lymphocyte origin of Reed-Sternberg cells. Lancet. 1974 Mar 2;1(7853):368–368. doi: 10.1016/s0140-6736(74)93138-9. [DOI] [PubMed] [Google Scholar]
- Casey T. T., Olson S. J., Cousar J. B., Collins R. D. Immunophenotypes of Reed-Sternberg cells: a study of 19 cases of Hodgkin's disease in plastic-embedded sections. Blood. 1989 Dec;74(8):2624–2628. [PubMed] [Google Scholar]
- Chittal S. M., Caverivière P., Schwarting R., Gerdes J., Al Saati T., Rigal-Huguet F., Stein H., Delsol G. Monoclonal antibodies in the diagnosis of Hodgkin's disease. The search for a rational panel. Am J Surg Pathol. 1988 Jan;12(1):9–21. doi: 10.1097/00000478-198801000-00002. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Curran R. C., Jones E. L. Dendritic cells and B lymphocytes in Hodgkin's disease. Lancet. 1977 Aug 13;2(8033):349–349. doi: 10.1016/s0140-6736(77)91502-1. [DOI] [PubMed] [Google Scholar]
- Delsol G., Meggetto F., Brousset P., Cohen-Knafo E., al Saati T., Rochaix P., Gorguet B., Rubin B., Voigt J. J., Chittal S. Relation of follicular dendritic reticulum cells to Reed-Sternberg cells of Hodgkin's disease with emphasis on the expression of CD21 antigen. Am J Pathol. 1993 Jun;142(6):1729–1738. [PMC free article] [PubMed] [Google Scholar]
- Drexler H. G., Jones D. B., Diehl V., Minowada J. Is the Hodgkin cell a T- or B-lymphocyte? Recent evidence from geno- and immunophenotypic analysis and in-vitro cell lines. Hematol Oncol. 1989 Mar-Apr;7(2):95–113. doi: 10.1002/hon.2900070202. [DOI] [PubMed] [Google Scholar]
- Drexler H. G., Leber B. F. The nature of the Hodgkin cell. Report of the First International Symposium on Hodgkin's Lymphoma, Köln, Federal Republic of Germany, October 2-3, 1987. Blut. 1988 Mar;56(3):135–137. doi: 10.1007/BF00320020. [DOI] [PubMed] [Google Scholar]
- Duh F. M., Latif F., Weng Y., Geil L., Modi W., Stackhouse T., Matsumura F., Duan D. R., Linehan W. M., Lerman M. I. cDNA cloning and expression of the human homolog of the sea urchin fascin and Drosophila singed genes which encodes an actin-bundling protein. DNA Cell Biol. 1994 Aug;13(8):821–827. doi: 10.1089/dna.1994.13.821. [DOI] [PubMed] [Google Scholar]
- Fisher R. I., Bostick-Bruton F., Sauder D. N., Scala G., Diehl V. Neoplastic cells obtained from Hodgkin's disease are potent stimulators of human primary mixed lymphocyte cultures. J Immunol. 1983 Jun;130(6):2666–2670. [PubMed] [Google Scholar]
- Freudenthal P. S., Steinman R. M. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7698–7702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell K., Pringle J. H., Hansmann M. L., Lorenzen J., Colloby P., Lauder I., Fischer R. Demonstration of light chain mRNA in Hodgkin's disease. J Pathol. 1993 Oct;171(2):137–143. doi: 10.1002/path.1711710211. [DOI] [PubMed] [Google Scholar]
- Hsu P. L., Hsu S. M. Identification of an Mr 70,000 antigen associated with Reed-Sternberg cells and interdigitating reticulum cells. Cancer Res. 1990 Jan 15;50(2):350–357. [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L. Aberrant expression of T cell and B cell markers in myelocyte/monocyte/histiocyte-derived lymphoma and leukemia cells. Is the infrequent expression of T/B cell markers sufficient to establish a lymphoid origin for Hodgkin's Reed-Sternberg cells? Am J Pathol. 1989 Jan;134(1):203–212. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L., Lo S. S., Wu K. K. Expression of prostaglandin H synthase (cyclooxygenase) in Hodgkin's mononuclear and Reed-Sternberg cells. Functional resemblance between H-RS cells and histiocytes or interdigitating reticulum cells. Am J Pathol. 1988 Oct;133(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Huang L. C., Hsu P. L., Ge Z. H., Ho Y. S., Cuttita F., Mulshine J. Biochemical and ultrastructural study of Leu M1 antigen in Reed-Sternberg cells: comparison with granulocytes and interdigitating reticulum cells. J Natl Cancer Inst. 1986 Aug;77(2):363–370. [PubMed] [Google Scholar]
- Hsu S. M. The never-ending controversies in Hodgkin's disease. Blood. 1990 Apr 15;75(8):1742–1744. [PubMed] [Google Scholar]
- Hsu S. M., Xie S. S., Hsu P. L. Cultured Reed-Sternberg cells HDLM-1 and KM-H2 can be induced to become histiocytelike cells. H-RS cells are not derived from lymphocytes. Am J Pathol. 1990 Aug;137(2):353–367. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X. Expression of interleukin-1 in Reed-Sternberg cells and neoplastic cells from true histiocytic malignancies. Am J Pathol. 1986 Nov;125(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X., Hsu P. L., Lok M. S. Extracellular matrix does not induce the proliferation, but promotes the differentiation, of Hodgkin's cell line HDLM-1. Am J Pathol. 1987 Apr;127(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Ziemann K., Lammert H., Pileri S., Sabattini E., Stein H. Hodgkin's disease with monoclonal and polyclonal populations of Reed-Sternberg cells. N Engl J Med. 1995 Oct 5;333(14):901–906. doi: 10.1056/NEJM199510053331403. [DOI] [PubMed] [Google Scholar]
- Kadin M. E. Possible origin of the Reed-Sternberg cell from an interdigitating reticulum cell. Cancer Treat Rep. 1982 Apr;66(4):601–608. [PubMed] [Google Scholar]
- Kadin M. E., Stites D. P., Levy R., Warnke R. Exogenous immunoglobulin and the macrophage origin of Reed-Sternberg cells in Hodgkin's disease. N Engl J Med. 1978 Nov 30;299(22):1208–1214. doi: 10.1056/NEJM197811302992203. [DOI] [PubMed] [Google Scholar]
- Kamel O. W., Gelb A. B., Shibuya R. B., Warnke R. A. Leu 7 (CD57) reactivity distinguishes nodular lymphocyte predominance Hodgkin's disease from nodular sclerosing Hodgkin's disease, T-cell-rich B-cell lymphoma and follicular lymphoma. Am J Pathol. 1993 Feb;142(2):541–546. [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. S., Gartner S. "Sternberg-reed" giant cells of Hodgkin's Disease: cultivation in vitro, heterotransplantation, and characterization as neoplastic macrophages. Int J Cancer. 1977 Apr 15;19(4):511–525. doi: 10.1002/ijc.2910190412. [DOI] [PubMed] [Google Scholar]
- Kennedy I. C., Hart D. N., Colls B. M., Nimmo J. C., Willis D. A., Angus H. B. Nodular sclerosing, mixed cellularity and lymphocyte-depleted variants of Hodgkin's disease are probable dendritic cell malignancies. Clin Exp Immunol. 1989 Jun;76(3):324–331. [PMC free article] [PubMed] [Google Scholar]
- Korkolopoulou P., Cordell J., Jones M., Kaklamanis L., Tsenga A., Gatter K. C., Mason D. Y. The expression of the B-cell marker mb-1 (CD79a) in Hodgkin's disease. Histopathology. 1994 Jun;24(6):511–515. doi: 10.1111/j.1365-2559.1994.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Küppers R., Rajewsky K., Zhao M., Simons G., Laumann R., Fischer R., Hansmann M. L. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10962–10966. doi: 10.1073/pnas.91.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R., Kaplan H. S. Impaired lymphocyte function in untreated Hodgkin's disease. N Engl J Med. 1974 Jan 24;290(4):181–186. doi: 10.1056/NEJM197401242900402. [DOI] [PubMed] [Google Scholar]
- Louie D. C., Kant J. A., Brooks J. J., Reed J. C. Absence of t(14;18) major and minor breakpoints and of Bcl-2 protein overproduction in Reed-Sternberg cells of Hodgkin's disease. Am J Pathol. 1991 Dec;139(6):1231–1237. [PMC free article] [PubMed] [Google Scholar]
- Lukes R. J. Criteria for involvement of lymph node, bone marrow, spleen, and liver in Hodgkin's disease. Cancer Res. 1971 Nov;31(11):1755–1767. [PubMed] [Google Scholar]
- Mosialos G., Birkenbach M., Ayehunie S., Matsumura F., Pinkus G. S., Kieff E., Langhoff E. Circulating human dendritic cells differentially express high levels of a 55-kd actin-bundling protein. Am J Pathol. 1996 Feb;148(2):593–600. [PMC free article] [PubMed] [Google Scholar]
- Mosialos G., Yamashiro S., Baughman R. W., Matsudaira P., Vara L., Matsumura F., Kieff E., Birkenbach M. Epstein-Barr virus infection induces expression in B lymphocytes of a novel gene encoding an evolutionarily conserved 55-kilodalton actin-bundling protein. J Virol. 1994 Nov;68(11):7320–7328. doi: 10.1128/jvi.68.11.7320-7328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady J. T., Stewart S., Lowrey J., Howie S. E., Krajewski A. S. CD40 expression in Hodgkin's disease. Am J Pathol. 1994 Jan;144(1):21–26. [PMC free article] [PubMed] [Google Scholar]
- Payne S. V., Newell D. G., Jones D. B., Wright D. H. The Reed-Sternberg cell/lymphocyte interaction: ultrastructure and characteristics of binding. Am J Pathol. 1980 Jul;100(1):7–24. [PMC free article] [PubMed] [Google Scholar]
- Payne S. V., Wright D. H., Jones K. J., Judd M. A. Macrophage origin of Reed-Sternberg cells: an immunohistochemical study. J Clin Pathol. 1982 Feb;35(2):159–166. doi: 10.1136/jcp.35.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., Lones M., Shintaku I. P., Said J. W. Immunohistochemical detection of Epstein-Barr virus-encoded latent membrane protein in Reed-Sternberg cells and variants of Hodgkin's disease. Mod Pathol. 1994 May;7(4):454–461. [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Hodgkin's disease, lymphocyte predominance type, nodular--a distinct entity? Unique staining profile for L&H variants of Reed-Sternberg cells defined by monoclonal antibodies to leukocyte common antigen, granulocyte-specific antigen, and B-cell-specific antigen. Am J Pathol. 1985 Jan;118(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Hodgkin's disease, lymphocyte predominance type, nodular--further evidence for a B cell derivation. L & H variants of Reed-Sternberg cells express L26, a pan B cell marker. Am J Pathol. 1988 Nov;133(2):211–217. [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., Thomas P., Said J. W. Leu-M1--a marker for Reed-Sternberg cells in Hodgkin's disease. An immunoperoxidase study of paraffin-embedded tissues. Am J Pathol. 1985 May;119(2):244–252. [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., Posner M. R., Schlossman S. F. In situ immunologic characterization of cellular constituents in lymph nodes and spleens involved by Hodgkin's disease. Blood. 1982 Feb;59(2):226–232. [PubMed] [Google Scholar]
- Poppema S., De Jong B., Atmosoerodjo J., Idenburg V., Visser L., De Ley L. Morphologic, immunologic, enzymehistochemical and chromosomal analysis of a cell line derived from Hodgkin's disease. Evidence for a B-cell origin of Sternberg-Reed cells. Cancer. 1985 Feb 15;55(4):683–690. doi: 10.1002/1097-0142(19850215)55:4<683::aid-cncr2820550402>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Roth J., Daus H., Trümper L., Gause A., Salamon-Looijen M., Pfreundschuh M. Detection of immunoglobulin heavy-chain gene rearrangement at the single-cell level in malignant lymphomas: no rearrangement is found in Hodgkin and Reed-Sternberg cells. Int J Cancer. 1994 Jun 15;57(6):799–804. doi: 10.1002/ijc.2910570607. [DOI] [PubMed] [Google Scholar]
- Said J. W., Sassoon A. F., Shintaku I. P., Kurtin P. J., Pinkus G. S. Absence of bcl-2 major breakpoint region and JH gene rearrangement in lymphocyte predominance Hodgkin's disease. Results of Southern blot analysis and polymerase chain reaction. Am J Pathol. 1991 Feb;138(2):261–264. [PMC free article] [PubMed] [Google Scholar]
- Schmid C., Pan L., Diss T., Isaacson P. G. Expression of B-cell antigens by Hodgkin's and Reed-Sternberg cells. Am J Pathol. 1991 Oct;139(4):701–707. [PMC free article] [PubMed] [Google Scholar]
- Sinkovics J. G. Hodgkin's disease revisited: Reed-Sternberg cells as natural hybridomas. Crit Rev Immunol. 1991;11(1):33–63. [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Strauchen J. A., Dimitriu-Bona A. Immunopathology of Hodgkin's disease. Characterization of Reed-Sternberg cells with monoclonal antibodies. Am J Pathol. 1986 May;123(2):293–300. [PMC free article] [PubMed] [Google Scholar]
- Stuart A. E., Williams A. R., Habeshaw J. A. Rosetting and other reactions of the Reed-Sternberg cell. J Pathol. 1977 Jun;122(2):81–90. doi: 10.1002/path.1711220205. [DOI] [PubMed] [Google Scholar]
- Söderström K. O., Rinne R., Hopsu-Havu V. K., Järvinen M., Rinne A. Hodgkin's disease: a malignancy of follicular dendritic cells? Lancet. 1994 Feb 12;343(8894):422–423. doi: 10.1016/s0140-6736(94)91260-2. [DOI] [PubMed] [Google Scholar]
- Tamaru J., Hummel M., Zemlin M., Kalvelage B., Stein H. Hodgkin's disease with a B-cell phenotype often shows a VDJ rearrangement and somatic mutations in the VH genes. Blood. 1994 Aug 1;84(3):708–715. [PubMed] [Google Scholar]
- Weiss L. M., Chang K. L. Molecular biologic studies of Hodgkin's disease. Semin Diagn Pathol. 1992 Nov;9(4):272–278. [PubMed] [Google Scholar]
- Yamashiro-Matsumura S., Matsumura F. Intracellular localization of the 55-kD actin-bundling protein in cultured cells: spatial relationships with actin, alpha-actinin, tropomyosin, and fimbrin. J Cell Biol. 1986 Aug;103(2):631–640. doi: 10.1083/jcb.103.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro-Matsumura S., Matsumura F. Purification and characterization of an F-actin-bundling 55-kilodalton protein from HeLa cells. J Biol Chem. 1985 Apr 25;260(8):5087–5097. [PubMed] [Google Scholar]