Abstract
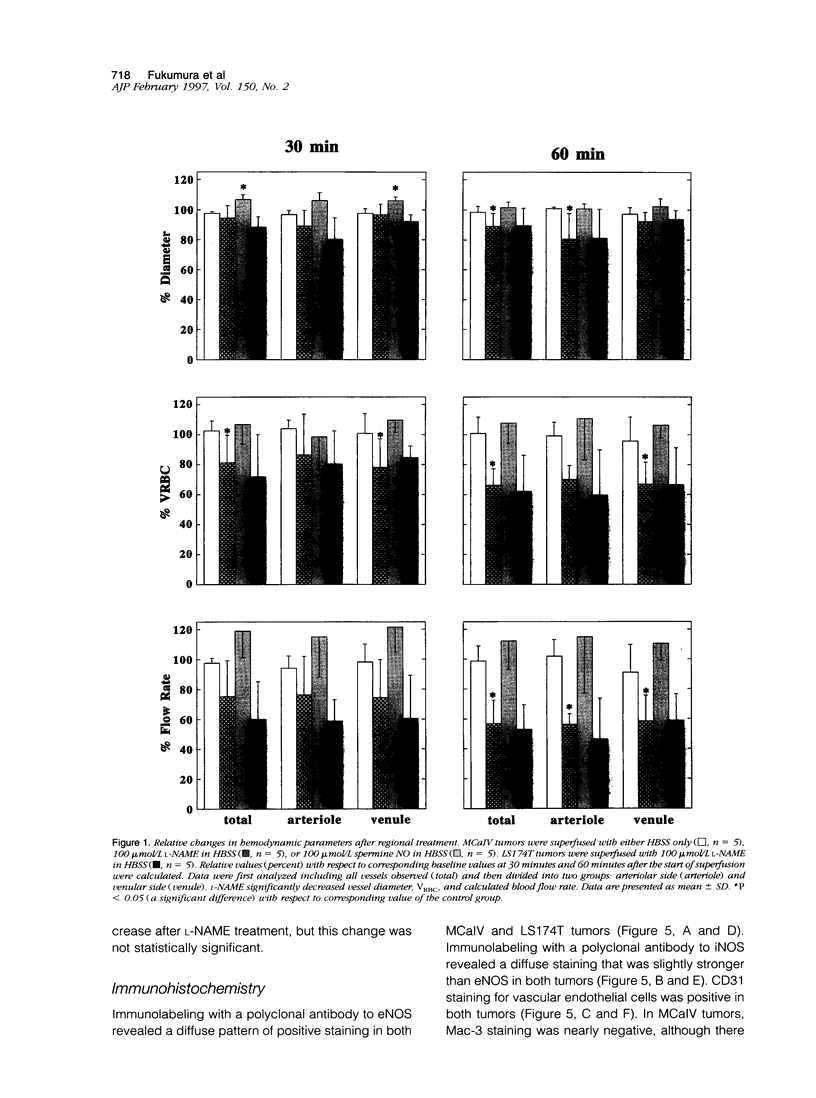
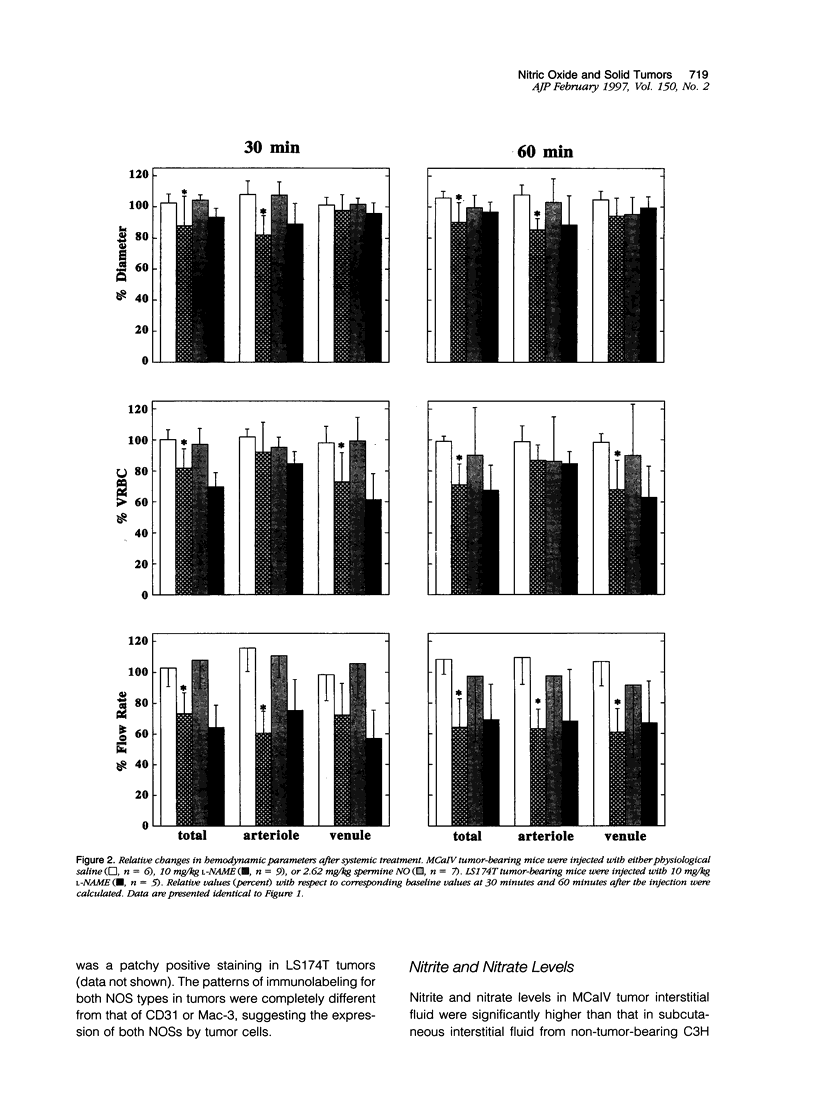
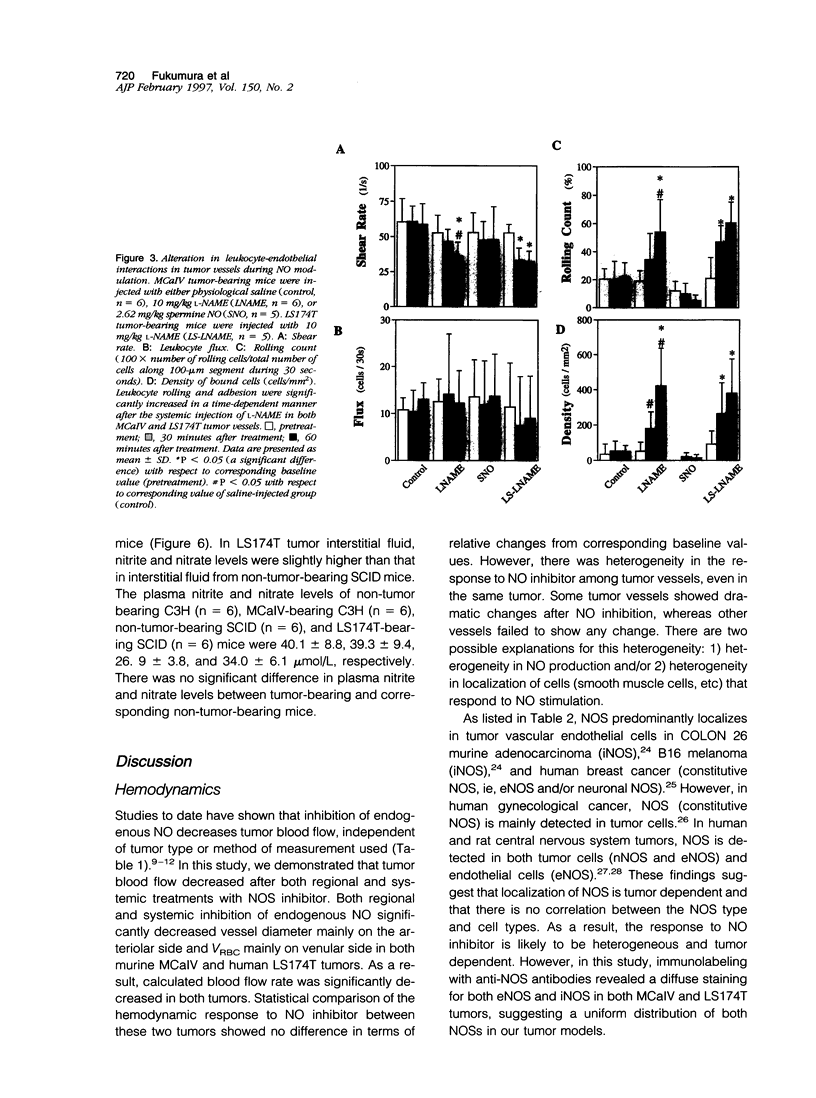
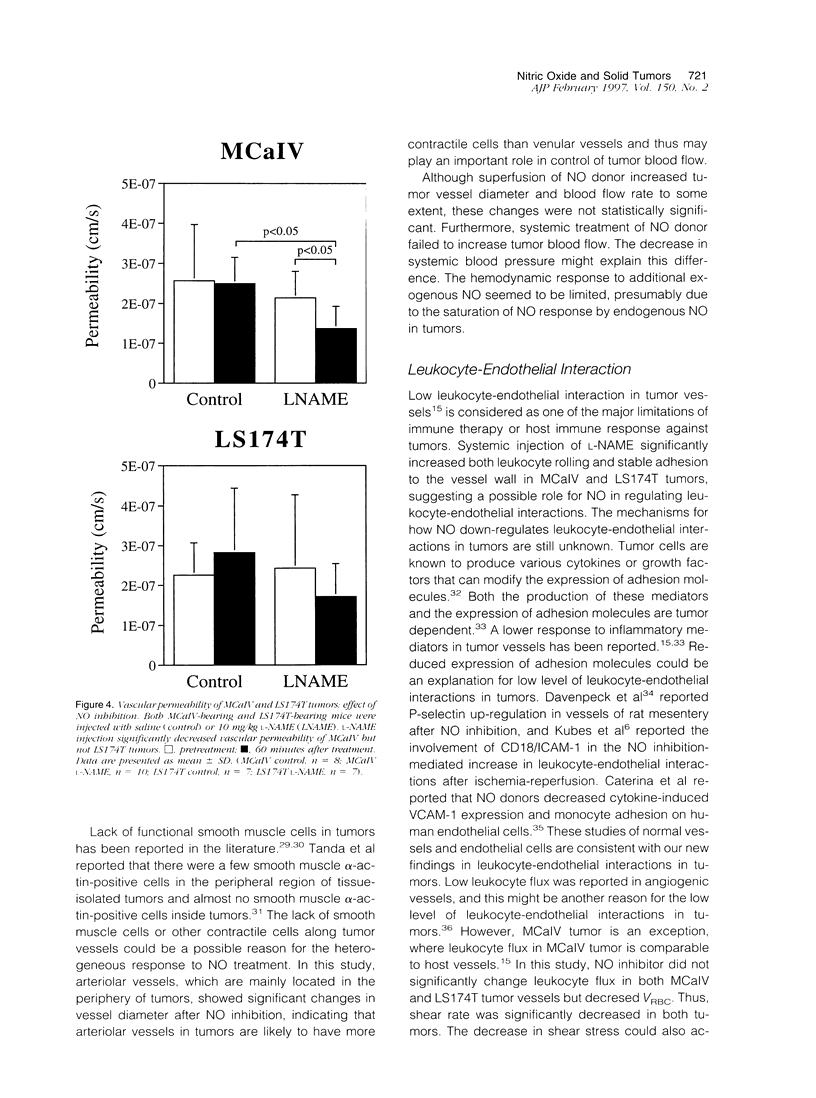
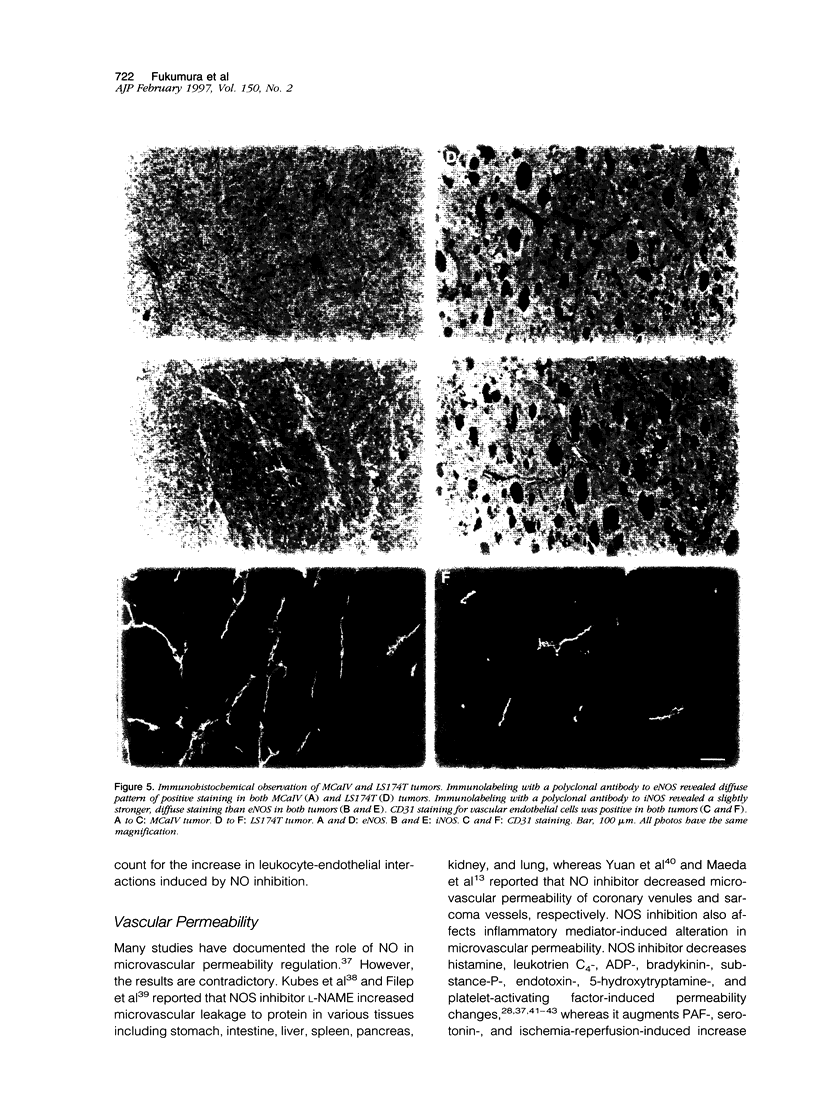
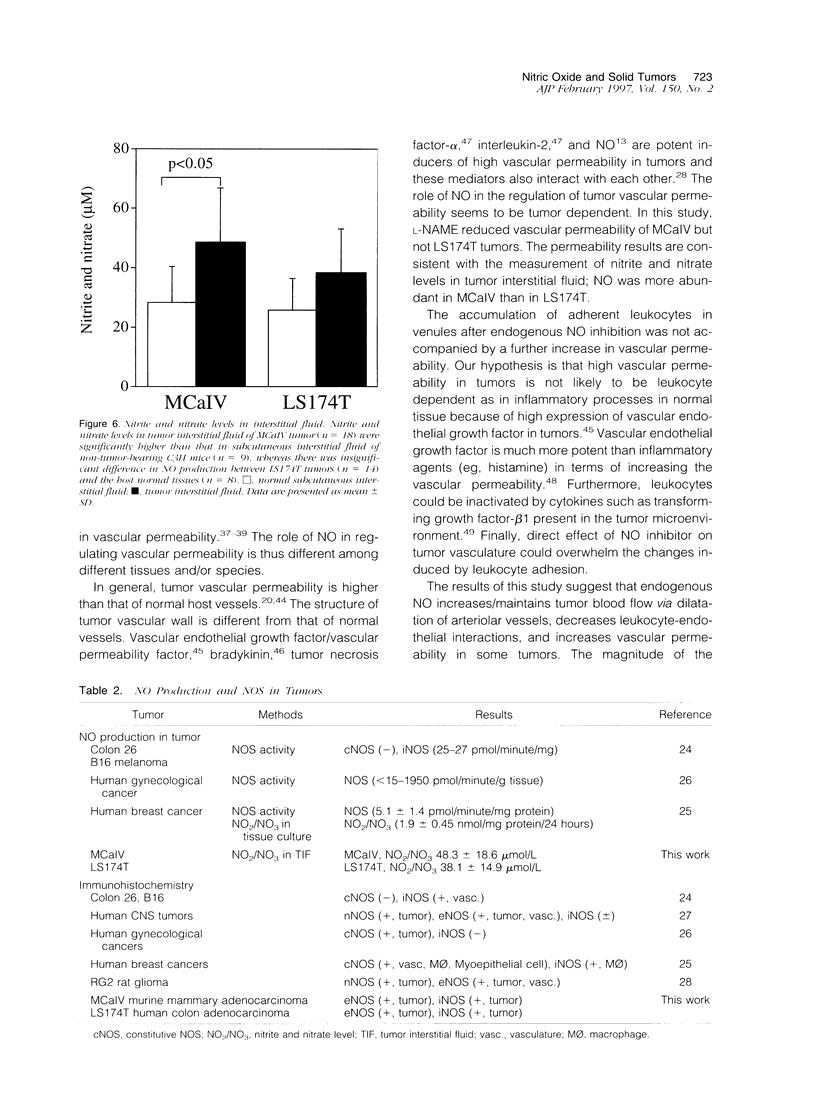
The present study was designed to define the role of nitric oxide (NO) in tumor microcirculation, through the direct intravital microcirculatory observations after administration of NO synthase (NOS) inhibitor and NO donor both regionally and systemically. More specifically, we tested the following hypotheses: 1) endogenous NO derived from tumor vascular endothelium and/or tumor cells increases and/or maintains tumor blood flow, decreases leukocyte-endothelial interactions, and increases vascular permeability, 2) exogenous NO can increase tumor blood flow via vessel dilatation and decrease leukocyte-endothelial interactions, and 3) NO production and tissue responses to NO are tumor dependent. To this end, a murine mammary adenocarcinoma (MCaIV) and a human colon adenocarcinoma (LS174T) were implanted in the dorsal skinfold chamber in C3H and severe combined immunodeficient mice, respectively, and observed by means of intravital fluorescence microscopy. Both regional and systemic inhibition of endogenous NO by N omega-nitro-L-arginine methyl ester (L-NAME; 100 mumol/L superfusion or 10 mg/kg intravenously) significantly decreased vessel diameter and local blood flow rate. The diameter change was dominant on the arteriolar side. Superfusion of NO donor (spermine NO, 100 mumol/L) increased tumor vessel diameter and flow rate, whereas systemic injection of spermine NO (2.62 mg/kg) had no significant effect on these parameters. Rolling and stable adhesion of leukocytes were significantly increased by intravenous injection of L-NAME. In untreated animals, both MCaIV and LS174T tumor vessels were leaky to albumin. Systemic NO inhibition significantly attenuated tumor vascular permeability of MCaIV but not of LS174T tumor. Immunohistochemical studies, using polyclonal antibodies to endothelial NOS and inducible NOS, revealed a diffuse pattern of positive labeling in both MCaIV and LS174T tumors. Nitrite and nitrate levels in tumor interstitial fluid of MCaIV but not of LS174T were significantly higher than that in normal subcutaneous interstitial fluid. These results support our hypotheses regarding the microcirculatory response to NO in tumors. Modulation of NO level in tumors is a potential strategy for altering tumor hemodynamics and thus improving oxygen, drug, gene vector, and effector cell delivery to solid tumors.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
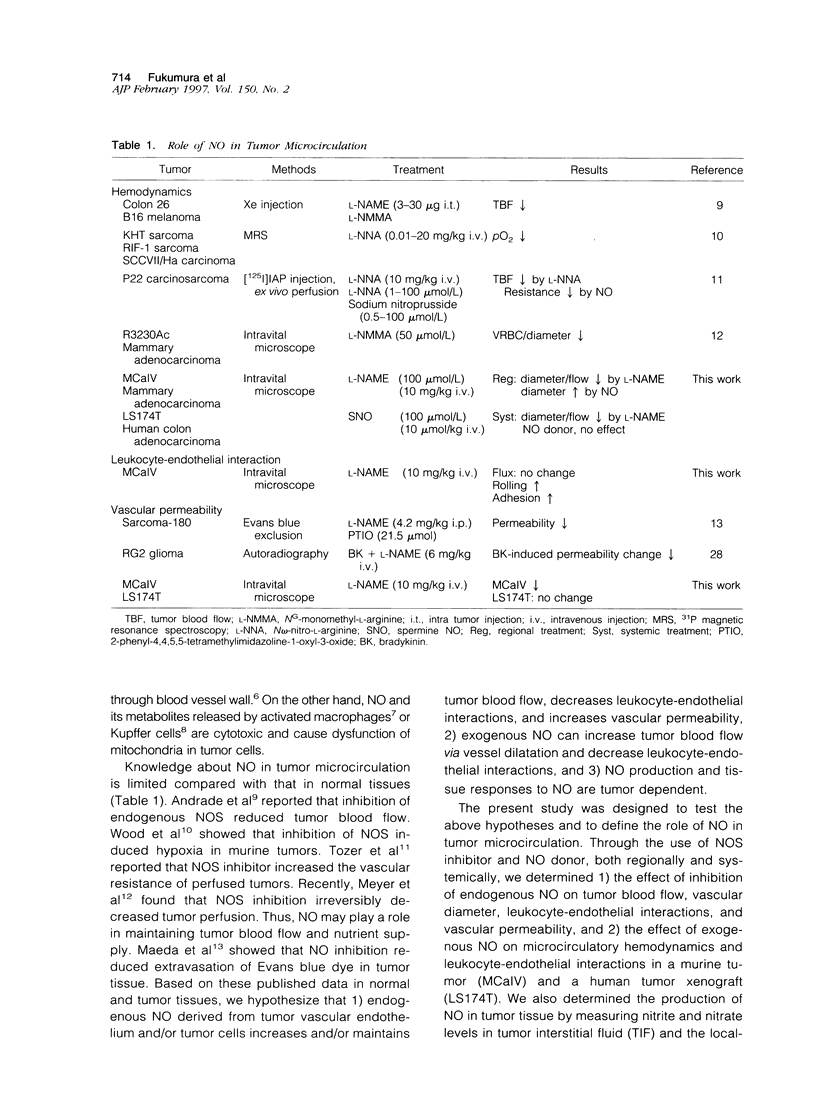
- Andrade S. P., Hart I. R., Piper P. J. Inhibitors of nitric oxide synthase selectively reduce flow in tumor-associated neovasculature. Br J Pharmacol. 1992 Dec;107(4):1092–1095. doi: 10.1111/j.1476-5381.1992.tb13412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuglia S., Colantuoni A., Intaglietta M. Capillary reperfusion after L-arginine, L-NMMA, and L-NNA treatment in cheek pouch microvasculature. Microvasc Res. 1995 Sep;50(2):162–174. doi: 10.1006/mvre.1995.1050. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Evans S. M., Laszlo F., Whittle B. J., Moncada S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br J Pharmacol. 1993 Nov;110(3):1189–1195. doi: 10.1111/j.1476-5381.1993.tb13940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizel D. M., Klitzman B., Cook J. M., Edwards J., Rosner G., Dewhirst M. W. A comparison of tumor and normal tissue microvascular hematocrits and red cell fluxes in a rat window chamber model. Int J Radiat Oncol Biol Phys. 1993 Jan 15;25(2):269–276. doi: 10.1016/0360-3016(93)90348-y. [DOI] [PubMed] [Google Scholar]
- Buttery L. D., Springall D. R., Andrade S. P., Riveros-Moreno V., Hart I., Piper P. J., Polak J. M. Induction of nitric oxide synthase in the neo-vasculature of experimental tumours in mice. J Pathol. 1993 Dec;171(4):311–319. doi: 10.1002/path.1711710412. [DOI] [PubMed] [Google Scholar]
- Cobbs C. S., Brenman J. E., Aldape K. D., Bredt D. S., Israel M. A. Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res. 1995 Feb 15;55(4):727–730. [PubMed] [Google Scholar]
- Davenpeck K. L., Gauthier T. W., Lefer A. M. Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology. 1994 Oct;107(4):1050–1058. doi: 10.1016/0016-5085(94)90229-1. [DOI] [PubMed] [Google Scholar]
- De Caterina R., Libby P., Peng H. B., Thannickal V. J., Rajavashisth T. B., Gimbrone M. A., Jr, Shin W. S., Liao J. K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995 Jul;96(1):60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellian M., Witwer B. P., Salehi H. A., Yuan F., Jain R. K. Quantitation and physiological characterization of angiogenic vessels in mice: effect of basic fibroblast growth factor, vascular endothelial growth factor/vascular permeability factor, and host microenvironment. Am J Pathol. 1996 Jul;149(1):59–71. [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Brown L. F., Detmar M., Dvorak A. M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995 May;146(5):1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Ettinghausen S. E., Puri R. K., Rosenberg S. A. Increased vascular permeability in organs mediated by the systemic administration of lymphokine-activated killer cells and recombinant interleukin-2 in mice. J Natl Cancer Inst. 1988 Apr 6;80(3):177–188. doi: 10.1093/jnci/80.3.177. [DOI] [PubMed] [Google Scholar]
- Filep J. G., Földes-Filep E. Modulation by nitric oxide of platelet-activating factor-induced albumin extravasation in the conscious rat. Br J Pharmacol. 1993 Dec;110(4):1347–1352. doi: 10.1111/j.1476-5381.1993.tb13967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii E., Irie K., Uchida Y., Tsukahara F., Muraki T. Possible role of nitric oxide in 5-hydroxytryptamine-induced increase in vascular permeability in mouse skin. Naunyn Schmiedebergs Arch Pharmacol. 1994 Oct;350(4):361–364. doi: 10.1007/BF00178952. [DOI] [PubMed] [Google Scholar]
- Fukumura D., Salehi H. A., Witwer B., Tuma R. F., Melder R. J., Jain R. K. Tumor necrosis factor alpha-induced leukocyte adhesion in normal and tumor vessels: effect of tumor type, transplantation site, and host strain. Cancer Res. 1995 Nov 1;55(21):4824–4829. [PubMed] [Google Scholar]
- Fukumura D., Yonei Y., Kurose I., Saito H., Ohishi T., Higuchi H., Miura S., Kato S., Kimura H., Ebinuma H. Role in nitric oxide in Kupffer cell-mediated hepatoma cell cytotoxicity in vitro and ex vivo. Hepatology. 1996 Jul;24(1):141–149. doi: 10.1053/jhep.1996.v24.pm0008707254. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Jain R. K., Koenig G. C., Dellian M., Fukumura D., Munn L. L., Melder R. J. Leukocyte-endothelial adhesion and angiogenesis in tumors. Cancer Metastasis Rev. 1996 Jun;15(2):195–204. doi: 10.1007/BF00437472. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6(4):559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- Jenkins D. C., Charles I. G., Thomsen L. L., Moss D. W., Holmes L. S., Baylis S. A., Rhodes P., Westmore K., Emson P. C., Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Granger D. N. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992 Feb;262(2 Pt 2):H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- Kubes P. Nitric oxide affects microvascular permeability in the intact and inflamed vasculature. Microcirculation. 1995 Sep;2(3):235–244. doi: 10.3109/10739689509146769. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunig M., Yuan F., Menger M. D., Boucher Y., Goetz A. E., Messmer K., Jain R. K. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 1992 Dec 1;52(23):6553–6560. [PubMed] [Google Scholar]
- Lipowsky H. H., Zweifach B. W. Application of the "two-slit" photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978 Jan;15(1):93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- Maeda H., Noguchi Y., Sato K., Akaike T. Enhanced vascular permeability in solid tumor is mediated by nitric oxide and inhibited by both new nitric oxide scavenger and nitric oxide synthase inhibitor. Jpn J Cancer Res. 1994 Apr;85(4):331–334. doi: 10.1111/j.1349-7006.1994.tb02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Kimura M., Yamamoto T., Maeda H. Involvement of the kinin-generating cascade in enhanced vascular permeability in tumor tissue. Jpn J Cancer Res. 1988 Dec;79(12):1327–1334. doi: 10.1111/j.1349-7006.1988.tb01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melder R. J., Koenig G. C., Witwer B. P., Safabakhsh N., Munn L. L., Jain R. K. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med. 1996 Sep;2(9):992–997. doi: 10.1038/nm0996-992. [DOI] [PubMed] [Google Scholar]
- Meyer R. E., Shan S., DeAngelo J., Dodge R. K., Bonaventura J., Ong E. T., Dewhirst M. W. Nitric oxide synthase inhibition irreversibly decreases perfusion in the R3230Ac rat mammary adenocarcinoma. Br J Cancer. 1995 Jun;71(6):1169–1174. doi: 10.1038/bjc.1995.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992 Jul;145(3):201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- Nakano S., Matsukado K., Black K. L. Increased brain tumor microvessel permeability after intracarotid bradykinin infusion is mediated by nitric oxide. Cancer Res. 1996 Sep 1;56(17):4027–4031. [PubMed] [Google Scholar]
- Orucevic A., Lala P. K. NG-nitro-L-arginine methyl ester, an inhibitor of nitric oxide synthesis, ameliorates interleukin 2-induced capillary leakage and reduces tumour growth in adenocarcinoma-bearing mice. Br J Cancer. 1996 Jan;73(2):189–196. doi: 10.1038/bjc.1996.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez M. M., Quardt S. M., Kim D., Oshiro H., Minnicozzi M., Durán W. N. Platelet activating factor modulates microvascular permeability through nitric oxide synthesis. Microvasc Res. 1995 Sep;50(2):223–234. doi: 10.1006/mvre.1995.1055. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Smith W. B., Noack L., Khew-Goodall Y., Isenmann S., Vadas M. A., Gamble J. R. Transforming growth factor-beta 1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol. 1996 Jul 1;157(1):360–368. [PubMed] [Google Scholar]
- Sneddon J. M., Vane J. R. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick P. L., Ernst L. A., Tauriello E. W., Parker S. R., Mujumdar R. B., Mujumdar S. R., Clever H. A., Waggoner A. S. Cyanine dye labeling reagents--carboxymethylindocyanine succinimidyl esters. Cytometry. 1990;11(3):418–430. doi: 10.1002/cyto.990110313. [DOI] [PubMed] [Google Scholar]
- Thomsen L. L., Lawton F. G., Knowles R. G., Beesley J. E., Riveros-Moreno V., Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994 Mar 1;54(5):1352–1354. [PubMed] [Google Scholar]
- Thomsen L. L., Miles D. W., Happerfield L., Bobrow L. G., Knowles R. G., Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995 Jul;72(1):41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer G. M., Prise V. E., Bell K. M. The influence of nitric oxide on tumour vascular tone. Acta Oncol. 1995;34(3):373–377. doi: 10.3109/02841869509093992. [DOI] [PubMed] [Google Scholar]
- Valenti G., Verbavatz J. M., Sabolić I., Ausiello D. A., Verkman A. S., Brown D. A basolateral CHIP28/MIP26-related protein (BLIP) in kidney principal cells and gastric parietal cells. Am J Physiol. 1994 Sep;267(3 Pt 1):C812–C820. doi: 10.1152/ajpcell.1994.267.3.C812. [DOI] [PubMed] [Google Scholar]
- Villringer A., Dirnagl U., Them A., Schürer L., Krombach F., Einhäupl K. M. Imaging of leukocytes within the rat brain cortex in vivo. Microvasc Res. 1991 Nov;42(3):305–315. doi: 10.1016/0026-2862(91)90064-i. [DOI] [PubMed] [Google Scholar]
- Wood P. J., Sansom J. M., Butler S. A., Stratford I. J., Cole S. M., Szabo C., Thiemermann C., Adams G. E. Induction of hypoxia in experimental murine tumors by the nitric oxide synthase inhibitor, NG-nitro-L-arginine. Cancer Res. 1994 Dec 15;54(24):6458–6463. [PubMed] [Google Scholar]
- Yuan F., Leunig M., Berk D. A., Jain R. K. Microvascular permeability of albumin, vascular surface area, and vascular volume measured in human adenocarcinoma LS174T using dorsal chamber in SCID mice. Microvasc Res. 1993 May;45(3):269–289. doi: 10.1006/mvre.1993.1024. [DOI] [PubMed] [Google Scholar]
- Yuan F., Salehi H. A., Boucher Y., Vasthare U. S., Tuma R. F., Jain R. K. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994 Sep 1;54(17):4564–4568. [PubMed] [Google Scholar]




