Abstract
Produced and released by the heart, oxytocin (OT) acts on its cardiac receptors to decrease the cardiac rate and force of contraction. We hypothesized that it might also be produced in the vasculature and regulate vascular tone. Consequently, we prepared acid extracts of the pulmonary artery and vena cava of male rats. OT concentrations in dog and sheep aortae were equivalent to those of rat aorta (2745 ± 180 pg/mg protein), indicating that it is present in the vasculature of several mammalian species. Reverse-phase HPLC of aorta and vena cava extracts revealed a single peak corresponding to the amidated OT nonapeptide. Reverse-transcribed PCR confirmed OT synthesis in these tissues. Using the selective OT receptor ligand compound VI, we detected a high number of OT-binding sites in the rat vena cava and aorta. Furthermore, OT receptor (OTR) mRNA was found in the vena cava, pulmonary vein, and pulmonary artery with lower levels in the aorta, suggesting vessel-specific OTR distribution. The abundance of OTR mRNA in the vena cava and pulmonary vein was associated with high atrial natriuretic peptide mRNA. In addition, we have demonstrated that diethylstilbestrol treatment of immature female rats increased OT significantly in the vena cava but not in the aorta and augmented OTR mRNA in both the aorta (4-fold) and vena cava (2-fold), implying regulation by estrogen. Altogether, these data suggest that the vasculature contains an intrinsic OT system, which may be involved in the regulation of vascular tone as well as vascular regrowth and remodeling.
Oxytocin (OT), a neurophyseal hormone, is known to play a role in lactation and parturition and in the central nervous system as a neurotransmitter involved in sex and maternal behavior (1). The presence of equivalent amounts of plasma OT as well as a similar number of oxytocinergic neurons in males as in females suggests a more general physiological and endocrine role of this hormone. In this context, OT emerges as a regulator of natriuresis and blood volume.
The mechanism of this regulation, which we proposed recently on the basis of blood volume expansion (VE) experiments in rats, involves OT release from the posterior pituitary gland into plasma, which is followed by atrial natriuretic peptide (ANP) release via activation of the OT receptors (OTRs) present in the heart (2). In effect, following blood VE, OT and ANP would produce a negative ionotropic and chronotropic response in the heart (3) that would rapidly reduce cardiac output and, thereby, effective circulating blood volume. Blood VE may also generate OT locally since the heart is a site of OT synthesis and OT secretion (4) as well as ANP secretion. Thus, the increased venous return to the heart following VE would stretch the cardiac myocytes activating OT release that would release ANP.
Then, as has been shown recently, the natriuretic effects of VE in the kidney are caused in part by OT acting on its receptors in the renal tubules to generate NO, leading to increased cGMP; the increased plasma ANP acting on renal ANP receptors would also activate guanylyl cyclase, generating additional cGMP (5). The increased concentrations of cGMP in both instances would close Na+ channels, decreasing tubular reabsorption and thereby increasing excretion of Na+.
Since the reduction in effective circulating blood volume that follows VE occurs more rapidly than can be accounted for by natriuresis, it has been accepted that the ANP released by VE also dilates blood vessels via activation of particulate guanylyl cyclase and liberation of cGMP, thereby producing a rapid reduction in effective circulating blood volume. In this context, OTR may also be important, since we have shown that OTR are present in cardiovascular tissues (2). Our previous data support this hypothesis and we already reported the presence of OTR mRNA in the rat aorta (2). Several groups have demonstrated that vascular cells synthesize a number of vasoactive peptides including C-type natriuretic peptide (CNP) (6) and arginine vasopressin (AVP) (7). Such observations, in addition to the fact that OT influences vascular tone (8) and transduces signals in porcine endothelial cell cultures (9) and human smooth muscle cells (10), led us to the hypothesis that rat blood vessels may also synthesize and store OT.
Materials and Methods
Animals.
Experiments were performed in accordance with Canadian Guidelines on Animal Care with the approval of the Bioethics Committee of Centre Hospitalier de l'Université de Montréal. Adult female (200–250 g) and immature female (21 days of age) Sprague Dawley rats were obtained from Charles River Breeding Laboratories. They were housed in a 12-h day-night cycle and tap water and rat chow were available ad libitum. The immature female rats were either implanted with a silastic tube containing diethylstilbestrol (DES; Steraloids, Newport, RI), an estrogen analogue, for 48 h as recently described (11). We selected this method based on the observations that acute treatment with female gonadal steroids induces OT gene expression in uteri of immature rats (12) but not in uteri of adult ovariectomized rats (13) and that DES gave a better response than estradiol in neonatal and adolescent rats (14). After sacrifice, the superior vena cava and the thoracic aorta were dissected and frozen in liquid nitrogen. Tissues were stored at −80°C until processed.
RIA.
The frozen tissue was homogenized in an ice-cold acid solution [1 M HCl/1% formic acid/1% trifluoroacetic acid (TFA)/1% NaCl]. The homogenates were centrifuged at 1,500 × g for 15 min at 4°C, and the supernatants were extracted with heat-activated Vycor glass beads. These were washed extensively with 60% acetone and then were activated for 1 h at 600°C before being stored at 100°C until used. The tissue homogenates (300 μl) were mixed with Vycor glass beads and were suspended in 50 mg/ml H2O (500 μl). The suspension was rotated vertically for 30 min at 4°C and then centrifuged for 3 min at low speed. The supernatant was discarded and the glass beads were washed with double-distilled water (1.5 ml). The adsorbed material was eluted with 60% acetonitrile in 0.05 M HCl. The solvent was evaporated by SpeedVac (Savant) centrifugation. OT was measured by RIA in tissue extracts as described previously (2). The antibody, specific for OT nonapeptide (generous gift from M. Morris, Wright State University, Dayton OH) and synthetic OT standards (Peninsula Laboratories) were used to measure tissue OT content. The labeling of OT with 125I-Na was performed with the lactoperoxidase method and iodinated tracer was purified by HPLC (Waters). Standard curves generated with 125I-OT demonstrated a sensitivity of 0.1 pg and linearity in the range of 0.25–200 pg/ml. The crossreactivity of the antibody (diluted 1:80,000) was <1% with AVP and vasotocin. The RIA was performed in RIA buffer [50 nM sodium phosphate (pH 7.5)/0.1 M EDTA/0.1% BSA/0.01% sodium azide]. Two hundred microliters of sample or standards (0–200 pg) was incubated with 100 μl of antibody for 24 h at 4°C. Then, 100 μl of 125I-OT (3,000 cpm) was added to each tube that was incubated for 48 h at 4°C. The separation of free from antibody-bound 125I-OT was performed by dextran-coated charcoal (4). The radioactivity in the supernatant then was measured in the gamma counter.
HPLC.
The dried acetone-extracted homogenates were dissolved in an aqueous solution of 20% acetonitrile containing 0.1% TFA and were applied to a C18 μBondapak column (4.6 × 250 mm) of a reverse-phase HPLC (Waters). The column was eluted with a linear gradient of 20–50% CH3CN/0.1% TFA at a flow rate of 1 ml/min. Sixty 1-ml fractions were collected and lyophilized in the Speed-Vac. Direct RIA after reconstitution of samples in RIA buffer determined the presence of OT in the HPLC fractions. The synthetic OT, as well as pituitary gland extracts, chromatographed under identical conditions served as standards.
Binding Studies.
Vascular tissues were homogenized using a Polytron (setting 6, twice for 10 s each time) in ice-cold 50 mM Tris-HCl buffer, pH 7.4, containing 3 mM MgCl2, 5 mM sucrose, 1 mM EDTA, and 0.5 mM PMSF (P-766; Sigma). The tissue homogenates were centrifuged at 4,000 × g for 10 min at 4°C; then, the supernatant was saved, and the pellet was rehomogenized and centrifuged as above. Both supernatants were combined and centrifuged at 30,000 × g for 40 min at 4°C. The pellet corresponding to the crude membrane fraction was resuspended in 0.5 ml Tris buffer solution (50 mM Tris·HCl, pH 7.4, and 10 mM MgCl2) and stored at −80°C. An iodinated, by the lactoperoxidase method, highly specific OT antagonist, [d(CH2)5Tyr(Me)2,Thr4,Tyr-NH29]OVT (Peninsula Laboratories), was used as a ligand for binding experiments. Optimal conditions (protein concentration and incubation time) of OT antagonist binding to heart membranes were determined in initial experiments. Consequently, the reaction mixture (250 μl) consisted of iodinated tracer (50 pM in 100 μl) and crude membranes (200 μg of protein) in Tris buffer. By adding increasing concentrations of cold ligand (10−12–10−6 M) to the reactions, we performed homologous competition experiments. Reactions were carried out in duplicate at room temperature for 1 h and stopped by addition of 3 ml of ice-cold Tris buffer, followed by rapid filtration through Whatman GF/C filters (Whatman) presoaked in 1% polyethylenimine (P-3143; Sigma). The filters were washed twice with 3 ml of cold 50 mM Tris-HCl buffer, pH 7.4, and then dried. Receptor-bound radioactivity was measured in a gamma counter (Cobra II Autogamma; Canberra-Packard, Mississauga, Ontario, Canada). Receptor-binding data were fitted with the aid of nonlinear regression analysis to obtain displacement curves and Bmax and Kd values using the GraphPad Prism (version 3.0; GraphPad, San Diego, CA) computer program.
PCR.
Total RNA was extracted from rat tissues by the Trizol reagent (GIBCO/BRL). Genomic DNA in the RNA extracts was removed by DNase treatment. The integrity of the RNA preparations was verified by gel electrophoresis and ethidium bromide staining of the gels. RNA concentrations were measured by UV spectrophotometry. First-strand cDNA was synthesized in a final volume (40 μl) containing first-strand buffer, 2 μg of rat vascular or uterine RNA, 2 μg of hexanucleotide primer (Amersham Pharmacia), and avian myeloblastosis virus reverse transcriptase (12 U/μg RNA; Life Sciences, St. Petersburg, FL). Ten microliters of the first-strand cDNA was then used for PCR amplification with different combinations of OT exon-specific oligonucleotide primers. The specific sequence of these primers has been described by Lefebvre et al. (15). A+ was a sense-strand primer corresponding to a sequence in exon 1, starting 3 bp downstream of the initiation codon. B+ and B− primers were sense- and antisense-strand primers located at the 5′ and 3′ ends of exon 2, respectively. C− was an antisense primer complementary to a sequence in exon 3, terminating at the stop codon. Amplification was performed for 36 cycles. Each cycle involved the following: 94°C for 1 min, 59°C for 1 min, and 72°C for 2 min followed by a 5-min final extension at 72°C. Ten microliters of the PCR reactions was electrophoresed on 1.5 agarose gel and transferred onto Hybond N+ nylon membranes (Amersham Pharmacia) by vacuum transfer in 1.5 M NaCl and 0.5 M NaOH. The probe used for Southern blot analysis of PCR products was an OT cDNA fragment corresponding to the sequences between primers B+ and B− on exon B of the OT gene. Control RT-PCR reactions were performed by omitting reverse transcriptase or RNA from the reaction mixture. Radioactive bands were counted using a PhosphorImager and ImageQuant (Molecular Dynamics) software.
The OTR RT-PCR assay used in this study has been described previously (2). Briefly, the primer pair used for PCR amplification was separated on the rat OTR gene by a large (over 12 kb) intron, hence preventing amplification of any contaminating genomic DNA. The sequence of the forward primer corresponded to bases 2821–2840 of the OTR gene (16). The reverse primer (bases 3927–3946) was complementary to a segment in the 3′ untranslated region. The cycle parameters for PCR were 1.5 min at 94°C, 1.5 min at 65°C, and 2 min at 72°C. The RT-PCR amplification of vasopressin V1A receptor mRNA was performed by RT-PCR according to protocol of Hirasawa et al. (17), and amplification of the ANP and CNP gene products was already published (18). For quantitation of the PCR reaction, 10 μCi of [32P]dCTP was added to the PCR buffer. The PCR products were electrophoresed in 2% agarose and transferred to a nylon membrane (Hybond N+; Amersham Pharmacia). A similar procedure was applied for amplification of rat GAPDH mRNA as described previously (2). The predicted size of the cDNA amplification product was 470 bp.
Results
OT Concentrations in the Cardiovascular System.
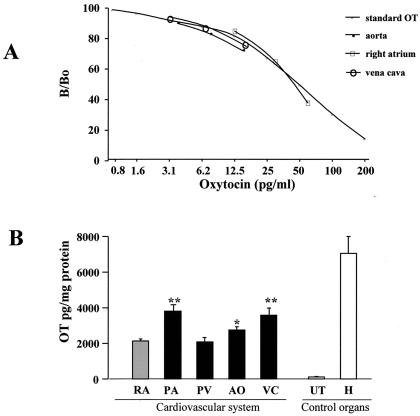
To determine whether OT exists in rat vascular tissue, we used RIA with an antibody that recognizes specifically biologically active, amidated nonapeptide OT. As shown in Fig. 1A, addition of increasing volumes of extracts from the vena cava, the aorta, and rat pituitary gland produced competition curves that were parallel to that of synthetic OT. This indicated that the peptide present in the vascular tissue homogenates is indistinguishable from synthetic OT. The OT concentration in the rat great vessels was comparable to that found in rat right atrium (2128 ± 114 pg/mg protein, mean ± SEM), the cardiac chamber richest in OT (Fig. 1B). With the exception of the pulmonary vein (2083 ± 37 pg/mg protein, P = 0.77), the acid extracts of rat pulmonary artery (4035 ± 319 pg/mg protein, P < 0.001), vena cava (3577 ± 403 pg/mg protein P < 0.001), and aorta (2745 ± 180 pg/mg protein P = 0.02) exceeded OT concentration in the right atrium. Interestingly, the OT concentrations in the large vessels were only 2- to 3-fold lower than the concentration of OT in the hypothalamus (7061 ± 950 pg/mg protein) and significantly higher (20-fold) than that in acid extracts of the uterus from female rats selected without regard to the stage of their estrous cycles (112 ± 29 pg/mg protein).
Figure 1.
(A) OT immunoreactivity of serial dilutions (1:80, 1:40, 1:20) of rat extracts form the vena cava and aorta as well as right atrium homogenates measured by RIA. Results are compared with the OT standard curve. The ordinate shows the ratio (expressed as percentage) of bound 125I-labeled OT in the presence (B) and absence (Bo) of synthetic oxytocin. (B) OT concentration in rat tissues obtained by RIA after prior extraction by Vycor heat-inactivated glass beads. OT concentration (mean ± SEM) is compared in right atrium (RA) vs. pulmonary artery (PA), pulmonary vein (PV), aorta (AO), and vena cava (VC). The uterus (UT) and hypothalamus (H) are used as a control organs.
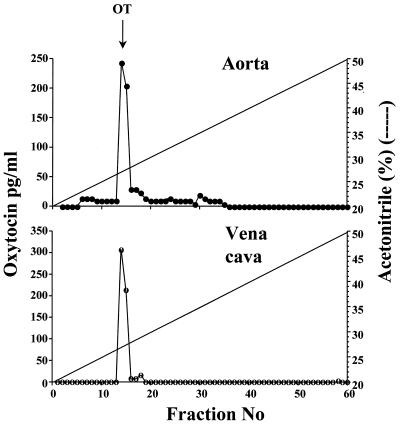
OT tissue concentrations in dog aorta (1749 ± 103 pg/mg protein) and sheep aorta (2012 ± 151 pg/mg protein) were equivalent to that in rat aorta, indicating that OT is not unique to rat vasculature. Reverse-phase HPLC purification of extracts of the rat and dog aorta and the rat vena cava revealed a single peak that coeluted with OT (Fig. 2).
Figure 2.
Reverse-phase HPLC elution of OT immunoreactivity in the rat aorta and vena cava. Tissue extracts, lyophilized and reconstituted in 20% acetonitrile in 0.1% TFA, were applied to a C18 μBondapak column and eluted with acetonitrile gradient (20–50%) in 0.1% TFA.
OT Synthesis in the Cardiovascular System.
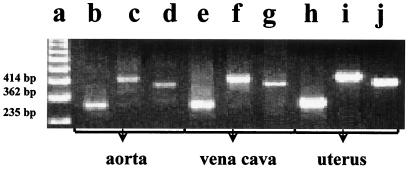
To determine whether OT detected in vascular tissue is synthesized locally, PCR amplification was used for identification of OT mRNA and to establish whether or not any difference exists between vascular and uterine transcripts. Three different pairs of exon-specific primers were used to amplify vena caval, aortic, and uterine cDNA. For each pair of primers, amplification of vascular and uterine cDNA generated products of identical size (Fig, 3). In each case, the size of the products obtained corresponded to the size predicted from the structure of the rat OT gene (16). Thus, RT-PCR analysis identified the coding region of the OT gene in the vascular beds studied.
OT-Binding Sites and Receptors in the Cardiovascular System.
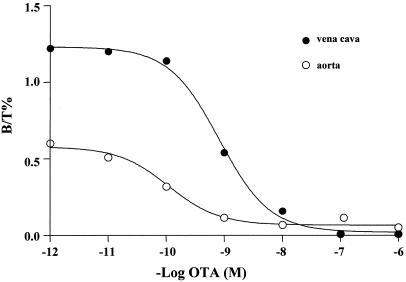
To investigate the sites of action of vascular OT, we performed binding assays on membranes prepared from the rat vena cava and aorta. OT-binding sites were detected via the iodinated OTR antagonist (125I-OT-ANT) (Fig. 4). Computer analysis of the competition binding curves revealed a single class of high-affinity binding sites in the rat vena cava (Kd = 0.78 nM) and aorta (Kd = 0.59 nM). The Kd values were not significantly different (P = 0.74). The number of 125I-OT-ANT-binding sites was higher in the rat vena caval membranes (96.9 ± 1.5 fmol/mg protein) than in the rat aorta (62.8 ± 1.5 fmol/mg protein, P < 0.001).
Figure 4.
125I-OT-ANT-binding sites in rat aorta and vena cava. Representative competition curves of 125I-OT-ANT binding to the aorta and vena cava membranes by unlabeled OT-ANT. Unlabeled OT-ANT was added at concentrations between 1 pM and 10 μM to the incubation medium containing 0.05 nM 125-OT-ANT and 50 μg of membrane proteins in final volume of 250 μl at 22°C for 60 min.
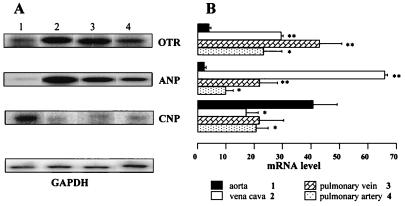
Further investigations showed that OTR mRNA is also present in vascular tissue. This was demonstrated by RT-PCR amplification (Fig. 5). There is only one intron (12 kb) in the coding region of the rat OTR gene, and the PCR amplified region contains the exon/intron boundary (16). The identical size of PCR products from the aorta, vena cava, and uterus indicates that there is no aberrant OTR protein from alternatively spliced transcripts in vascular tissue. To validate the use of this RT-PCR assay as a tool for the semiquantitative measurement of OTR mRNA, dose-response curves were established using different amounts of total RNA extracted from vascular tissues. The results showed that for the OTR mRNA, ANP mRNA, CNP mRNA, as well as the GAPDH mRNA assays, there was a steady increase in the response as doses were increased from 0.2 μg to 4 μg input RNA. Using this assay, we have shown that the expression of OTR mRNA, ANP mRNA, and CNP mRNA differed in the aorta on comparison with the other large vessels studied. As shown in Fig. 5, the higher expression of OTRs in the vena cava (P < 0.001) and pulmonary vein (P < 0.05) than in the aorta was associated with similar changes in abundance of ANP mRNA. In contrast, the CNP mRNA expression was significantly higher in the aorta than in the vena cava (P < 0.05) and pulmonary artery (P < 0.05). This implies a vessel-specific OTR distribution and possibly a vessel-specific action. On the other hand, there were no significant differences between the aorta and vena cava in the level of vasopressin V1 receptor mRNA, an alternative receptor site for 125I-OT binding (data not shown). Altogether, these results indicate that 125I-OT-ANT binding to the vena cava membranes reflects the presence of OTR.
Figure 5.
Distribution of OTR mRNA, ANP mRNA, and CNP mRNA in rat vessels as determined by semiquantitative RT-PCR. Total RNA (1 μg) was reverse transcribed and cDNA was exponentially amplified by PCR in the presence of gene-specific primers. (A) The PhosphorImager scan is presented after separation of radioactive PCR product by electrophoresis in 1.5% agarose. The results are verified to GAPDH mRNA PCR product used as internal standard. (B) The relative mRNA levels for OTR, ANP, and CNP in the vena cava (lane 2), pulmonary vein (lane 3), and pulmonary artery (lane 4) were compared with the level detected in the aorta (lane 1).
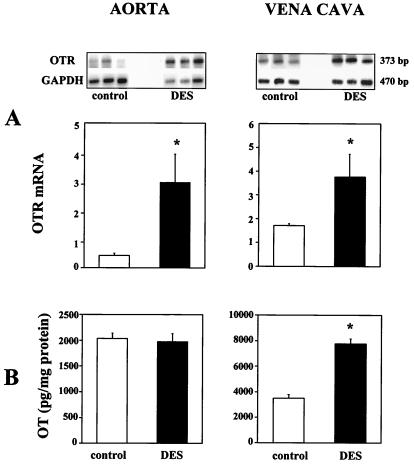
To investigate the effect of DES on OT and OTR and the regulation of the OT and OTR in the vena cava and aorta, immature rats were treated with DES. DES increased OTR mRNA 4-fold in the aorta and 2-fold in the vena cava as measured by semiquantitative RT-PCR (Fig. 6). DES treatment also increased OT in the vena cava from 3497 ± 350 to 7756 ± 445 pg/mg protein; however, it had no effect on the OT concentration in the aorta.
Figure 6.
Effects of treatment of DES on OTR mRNA and immunoreactive OT in the aorta and vena cava of immature female rats. (A) OTR mRNA was determined by semiquantitative RT-PCR. Total RNA (1 μg) was reverse transcribed and cDNA was exponentially amplified by PCR in the presence of gene-specific primers. PhosphoImager scan (upper panel) is presented after separation of radioactive PCR product by electrophoresis in 1.5% agarose. The bar graphs present the OTR mRNA level verified to GAPDH mRNA PCR product used as internal standard and calculated as a ratio of signal given by radioactive bands OTR mRNA PCR product (n = 4, *P < 0.05). (B) Comparison of OT content in the aorta and vena cava in control and DES-treated rats by RIA.
Discussion
This study shows that an intrinsic OT system sensitive to estrogenic regulation exists in the vasculature. Immunoreactive OT has been identified in rat, sheep, and dog vessels by specific RIA and characterized by HPLC. The RT-PCR analysis identified OT transcripts containing the coding sequence of the OT gene in the aorta and vena cava. The binding of membranes with 125I-OT-ANT and detection of OTR mRNA confirmed the presence of OTRs in the vasculature. Estrogen treatment enhanced OT and OTR gene expression in the vena cava, but only OTRs were increased in the aorta.
The vessels of the rat and other species contain significant amounts of OT immunologically equivalent with synthetic OT. Levels of vascular OT (2–4 ng/mg protein) are comparable to those obtained in other peripheral tissues, i.e., ovary (19) and thymus (20), and are only 2- to 4-fold lower than the levels found in the hypothalamus. Chromatography of the aortic and vena caval extracts on reverse-phase HPLC produced a peak that comigrated with pituitary extract and synthetic OT. These observations along with the finding that vessels express OT mRNA indicate that vascular OT is of local origin.
Our finding of vascular OT and OTR transcripts as well as earlier findings of vascular AVP (21) and V1 receptor (17) synthesis suggest that both neurophyseal hormones have a distinct physiological role in the control of vascular tone. Earlier studies minimized the significance of OTR in vascular beds, arguing that OT is a weaker constrictor than AVP in large vessels and in microvasculature and acts as a partial agonist of AVP (22, 23). The vasoconstrictor action of high concentrations of OT (22) may be caused by activation of V1 receptors. Since it occurs at supraphysiological doses of OT, we hypothesize that this is a pharmacological effect. Activation of OTR increased intracellular Ca2+ in smooth muscle cells from human aorta (10) and V1 receptors have been found in smooth muscle cells from human aorta as well. This may explain the vasoconstrictor action of high doses of OT (24).
Recently published data of Thibonnier et al. (25) revealed the existence of OTR but not AVP receptors in human vascular endothelial cell cultures from the aorta, umbilical vein, and pulmonary artery. These endothelial OT receptors produced a calcium-dependent stimulation of the NO pathway, leading to dilation in intact vessels. OT would generate NO that would activate guanylyl cyclase, leading to production of cGMP that would dilate the vascular smooth muscle. We hypothesize that the OTRs that we have demonstrated are located on the endothelial cells as would be predicted from these studies (9, 25). In recent experiments, administration of physiologically relevant doses of OT chronically to rats lowered blood pressure (26), results that suggest that the physiological action of OT is vasodilatory.
ANP is also synthesized and stored in granules in rat blood vessels. We have already shown that OT acts on its receptors in the heart (2, 3) and kidney (5) to release ANP. ANP acts on guanylyl cyclase to release cGMP that has a negative chrono- and ionotropic effect in the heart and adds to the natriuresis occurring in the kidney induced by OT. Therefore, we hypothesize that OT in the vessels may also act to release ANP that would in turn release cGMP that would have an additive effect along with that of NO to increase vasodilatation. OT-induced ANP and NO release may play a role in the capacitance of blood vessels as well. The combined effects would lower blood pressure. Thus, rapid vasodilatation following blood VE could be explained by the release of OT and ANP not only into the circulation but also locally in the vessels as a result of VE.
The mechanism of activation of OT release within the vessels has not been studied. It may be released by stretch or via parasympathetic stimulation of acetylcholine release that would act on muscarinic cholinergic receptors to increase intracellular Ca2+ concentrations within the cell to activate OT as well as NO release. The OT would also act to release ANP from secretory granules as well.
We have shown that treatment of immature female rats with estrogen increases OTR mRNA in the aorta and induces a coordinate response of OT mRNA and OTR mRNA in the vena cava. Thus, these results imply that the vascular effects of OT could be controlled by estrogen. Estradiol, but not progesterone or testosterone, increases Ca2+-dependent NO synthase activity (27) as well as increasing OTR (28). In addition, as we recently demonstrated, activation of OTR activates the NO pathway (5). Therefore, our results suggest that OT and OTR increased by estrogen stimulate the NO pathway in endothelial vascular cells, leading to dilation. The doses of DES used may not be physiological except during the elevated estrogen secretion during pregnancy. Therefore, further studies are needed using different doses of estradiol to determine the physiological significance of our findings.
In conclusion, our results have demonstrated that OT is locally synthesized and stored in the great vessels of the rat and can act on its receptors that are also synthesized in these vessels. We hypothesize that OT will be shown to play an important physiological role in control of vascular tone, as well as its already described role in control of cardiac function.
Figure 3.
PCR analysis of rat RNA from the aorta (b–d), vena cava (e–g), and uterus (h–j) for the expression of coding sequences of the OT gene. Ethidium bromide-stained agarose gel showing the PCR amplification products obtained with OT exon-specific primers using reverse-transcribed mRNA from rat aorta, vena cava, and uterus were used as control organs. a, 123-bp ladder of GIBCO/BRL; b, e, and h, 235-bp OT cDNA products of PCR from primers located on exons 1 and 2; c, f, and i, 414-bp OT cDNA products of PCR from primers located on exons 1 and 3; d, g, and j, 362-bp OT cDNA products of PCR from primers located on exons 2 and 3.
Acknowledgments
We express gratitude to Céline Coderre and Nathalie Charron for their technical assistance and to Dominique Poutrieux for her secretarial help. These studies were supported by Medical Research Council of Canada Grants MT-15049 (to M.J. and J.G.), MT-10337 (to J.G.), a grant from the Heart and Stroke Foundation of Canada (to J.G.), and Grant MH 51583 from the National Institute of Mental Health (to S.M.M.).
Abbreviations
- OT
oxytocin
- ANP
atrial natriuretic peptide
- OTR
OT receptor
- AVP
arginine vasopressin
- DES
diethylstibestrol
- TFA
trifluoroacetic acid
- 125I-OT-ANT
125I-labeled OT antagonist
- CNP
C-type natriuretic peptide
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.110137497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.110137497
References
- 1.Argiolas A, Gessa G L. Neurosci Biobehav Rev. 1991;15:217–231. doi: 10.1016/s0149-7634(05)80002-8. [DOI] [PubMed] [Google Scholar]
- 2.Gutkowska J, Jankowski M, Lambert C, Mukaddam-Daher S, Zingg H H, McCann S M. Proc Natl Acad Sci USA. 1997;94:11704–11709. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Favaretto A L, Ballejo G O, Albuquerque-Araujo W I, Gutkowska J, Antunes-Rodrigues J, McCann S M. Peptides. 1997;18:1377–1381. doi: 10.1016/s0196-9781(97)00209-x. [DOI] [PubMed] [Google Scholar]
- 4.Jankowski M, Hajjar F, Al Kawas S, Mukaddam-Daher S, Hoffman G, McCann S M, Gutkowska J. Proc Natl Acad Sci USA. 1998;95:14558–14563. doi: 10.1073/pnas.95.24.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soares T J, Coimbra T M, Martins A R, Pereira A G, Carnio E C, Branco L G, Albuquerque-Araujo W I, De Nucci G, Favaretto A L, Gutkowska J, et al. Proc Natl Acad Sci USA. 1999;96:278–283. doi: 10.1073/pnas.96.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon J S, Brody M J, Kasson B G. Am J Physiol. 1992;262:H799–H805. doi: 10.1152/ajpheart.1992.262.3.H799. [DOI] [PubMed] [Google Scholar]
- 8.Altura B M, Altura B T. Fed Proc. 1984;43:80–86. [PubMed] [Google Scholar]
- 9.Schini V B, Katusic Z S, Vanhoutte P M. J Pharmacol Exp Ther. 1990;255:994–1000. [PubMed] [Google Scholar]
- 10.Yazawa H, Hirasawa A, Horie K, Saita Y, Iida E, Honda K, Tsujimoto G. Br J Pharmacol. 1996;117:799–804. doi: 10.1111/j.1476-5381.1996.tb15263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noubani A, Farookhi R, Gutkowska J. Endocrinology. 2000;141:551–559. doi: 10.1210/endo.141.2.7305. [DOI] [PubMed] [Google Scholar]
- 12.Lefebvre D L, Farookhi R, Larcher A, Neculcea J, Zingg H H. Endocrinology. 1994;134:2556–2561. doi: 10.1210/endo.134.6.8194482. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi T, Liu C X, Saito H, Negoro H, Matsukawa S. J Endocrinol. 1995;146:81–85. doi: 10.1677/joe.0.1460081. [DOI] [PubMed] [Google Scholar]
- 14.Stack G, Gorski J. Endocrinology. 1983;112:2142–2146. doi: 10.1210/endo-112-6-2142. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre D L, Giaid A, Bennett H, Lariviere R, Zingg H H. Science. 1992;256:1553–1555. doi: 10.1126/science.1598587. [DOI] [PubMed] [Google Scholar]
- 16.Rozen F, Russo C, Banville D, Zingg H H. Proc Natl Acad Sci USA. 1995;92:200–204. doi: 10.1073/pnas.92.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirasawa A, Hashimoto K, Tsujimoto G. Eur J Pharmacol. 1994;267:71–75. doi: 10.1016/0922-4106(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 18.Jankowski M, Petrone C, Tremblay J, Gutkowska J. Regul Pept. 1996;62:53–61. doi: 10.1016/0167-0115(96)00004-3. [DOI] [PubMed] [Google Scholar]
- 19.Ivell R, Richter D. EMBO J. 1984;3:2351–2354. doi: 10.1002/j.1460-2075.1984.tb02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geenen V, Legros J J, Franchimont P, Baudrihaye M, Defresne M P, Boniver J. Science. 1986;232:508–511. doi: 10.1126/science.3961493. [DOI] [PubMed] [Google Scholar]
- 21.Simon J, Kasson B G. Hypertension. 1995;25:1030–1033. doi: 10.1161/01.hyp.25.5.1030. [DOI] [PubMed] [Google Scholar]
- 22.Manning M, Sawyer W H. J Recept Res. 1993;13:195–214. doi: 10.3109/10799899309073655. [DOI] [PubMed] [Google Scholar]
- 23.Jovanovic A, Jovanovic S, Tulic I, Grbovic L. Br J Pharmacol. 1997;121:1468–1474. doi: 10.1038/sj.bjp.0701273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burrell L M, Phillips P A, Rolls K A, Buxton B F, Johnston C I, Liu J J. Clin Sci. 1994;87:389–395. doi: 10.1042/cs0870389. [DOI] [PubMed] [Google Scholar]
- 25.Thibonnier M, Conarty D M, Preston J A, Plesnicher C L, Dweik R A, Erzurum S C. Endocrinology. 1999;140:1301–1309. doi: 10.1210/endo.140.3.6546. [DOI] [PubMed] [Google Scholar]
- 26.Petersson M, Alster P, Lundeberg T, Uvnas-Moberg K. Physiol Behav. 1996;60:1311–1315. doi: 10.1016/s0031-9384(96)00261-2. [DOI] [PubMed] [Google Scholar]
- 27.Weiner C P, Lizasoain I, Baylis S A, Knowles R G, Charles I G, Moncada S. Proc Natl Acad Sci USA. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grazzini E, Guillon G, Mouillac B, Zingg H H. Nature (London) 1998;392:509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]