Abstract
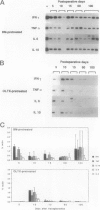
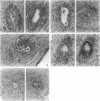
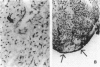
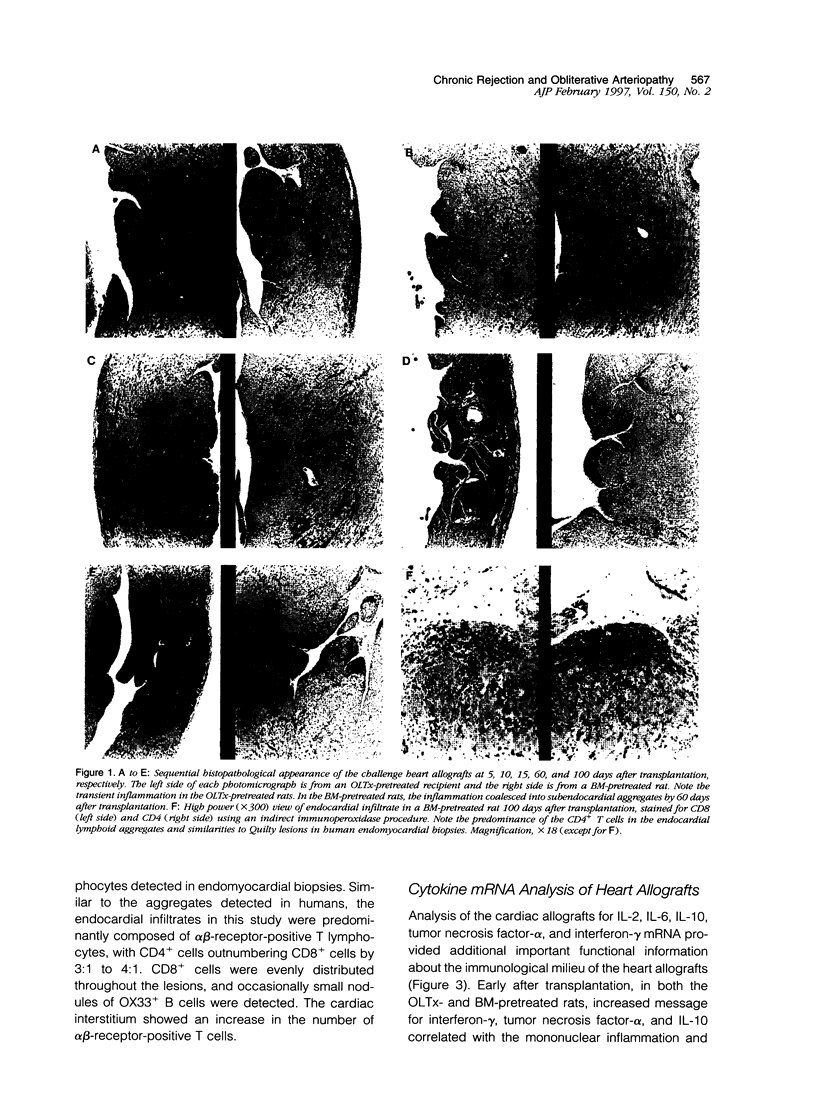
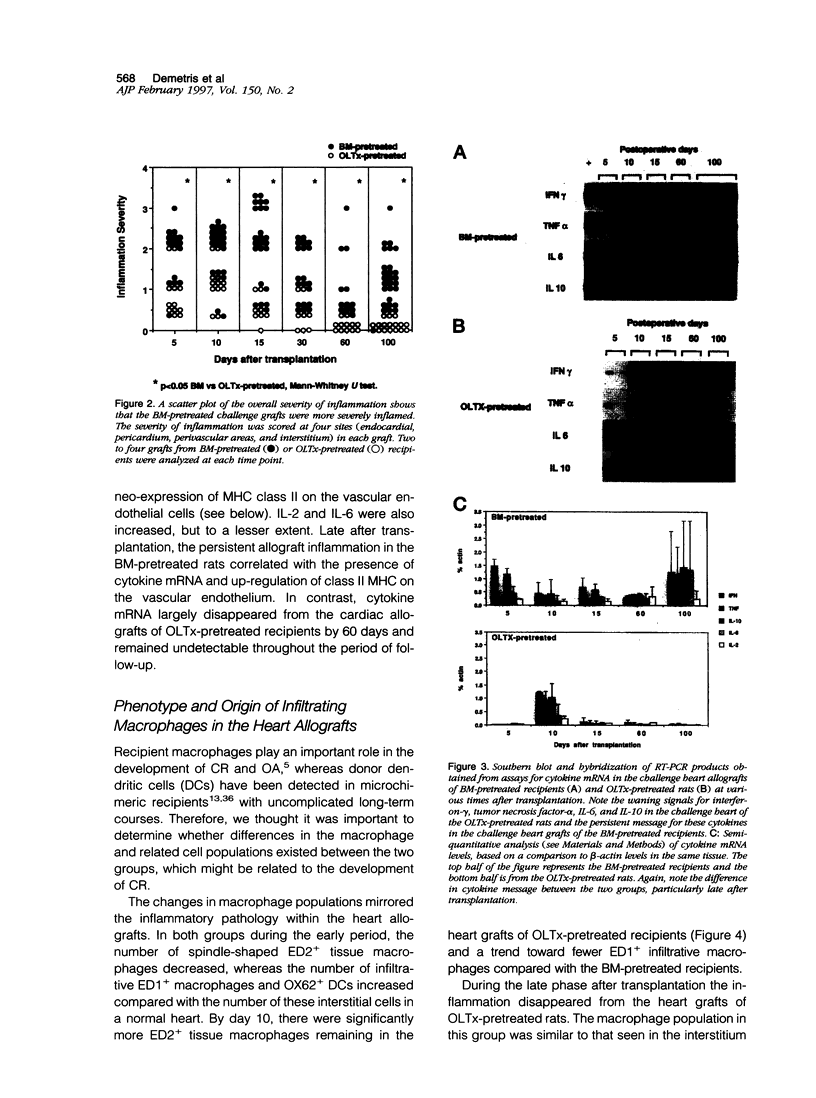
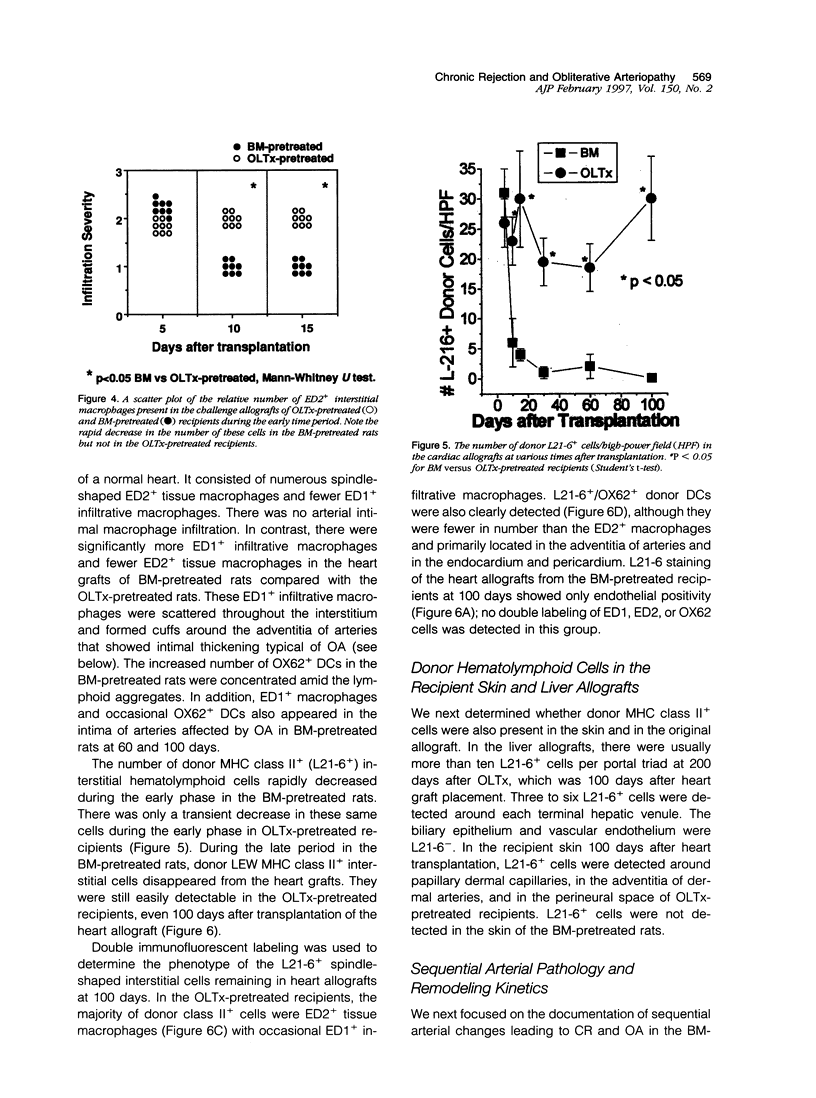
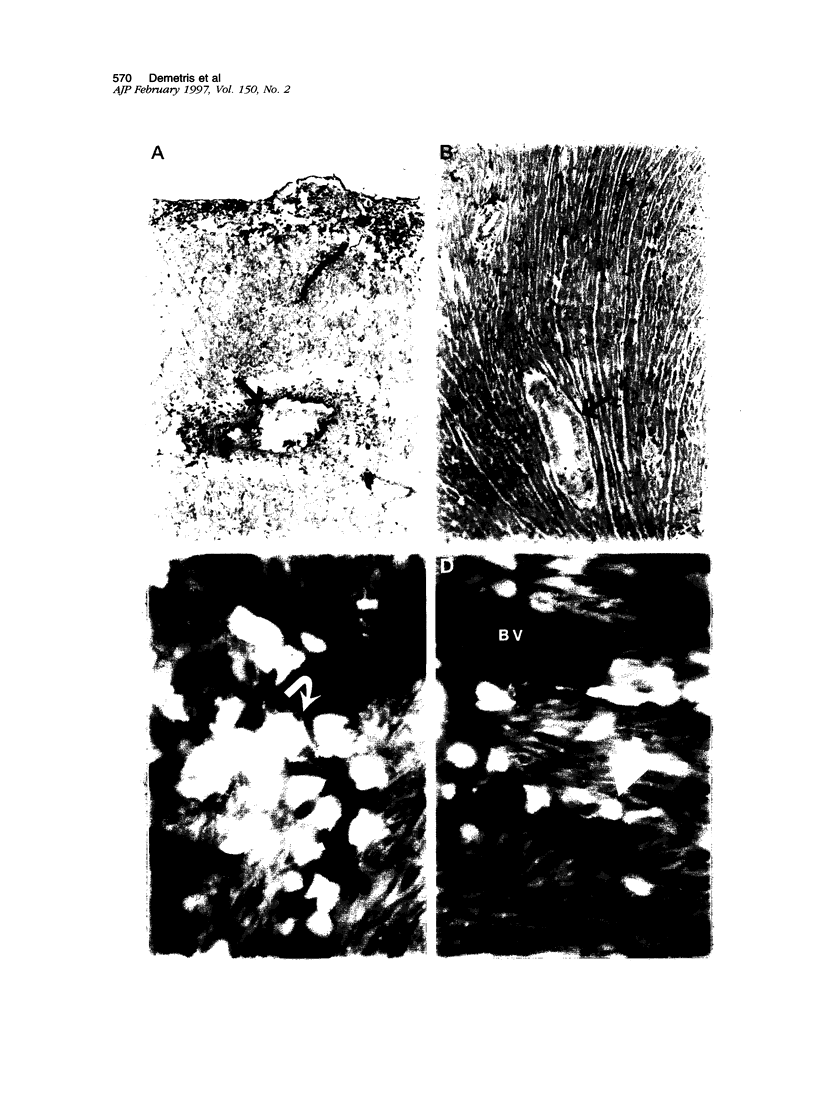
Sequential analysis of changes that lead to chronic rejection was undertaken in an animal model of chronic rejection and obliterative arteriopathy. Brown Norway rats are pretreated with a Lewis bone marrow infusion or a Lewis orthotopic liver allograft and a short course of immunosuppression. They are challenged 100 days later with a Lewis heterotopic heart graft without immunosuppression. The heart grafts in both groups undergo a transient acute rejection, but all rats are operationally tolerant; the heart grafts are accepted and remain beating for more than 100 days. Early arterial remodeling, marked by arterial bromodeoxyuridine incorporation, occurred in both groups between 5 and 30 days during the transient acute rejection. It coincided with the presence of interstitial (but not arterial intimal) inflammation and lymphatic disruption and resulted in mild intimal thickening. Significant arterial narrowing occurred only in the bone-marrow-pretreated rats between 60 and 100 days. It was associated with T lymphocyte and macrophage inflammation of the heart graft that accumulated in the endocardium and arterial intima and adventitia near draining lymphatics. There also was loss of passenger leukocytes from the heart graft, up-regulation of cytokine mRNA and major histocompatibility class II on the endothelium, and focal disruption of lymphatics. In contrast, long-surviving heart grafts from the Lewis orthotopic liver allograft pretreated group are near normal and freedom from chronic rejection in this group was associated with persistence of donor major histocompatibility class-II-positive hematolymphoid cells, including OX62+ donor dendritic cells. This study offers insights into two different aspects of chronic rejection: 1) possible mechanisms underlying the persistent immunological injury and 2) the association between immunological injury and the development of obliterative arteriopathy. Based on the findings, it is not unreasonable to raise the testable hypothesis that direct presentation of alloantigen by donor antigen-presenting cells is required for long-term, chronic-rejection-free allograft acceptance. In addition, chronic intermittent lymphatic disruption is implicated as a possible mechanism for the association between chronic interstitial allograft inflammation and the development of obliterative arteriopathy.
Full text
PDF


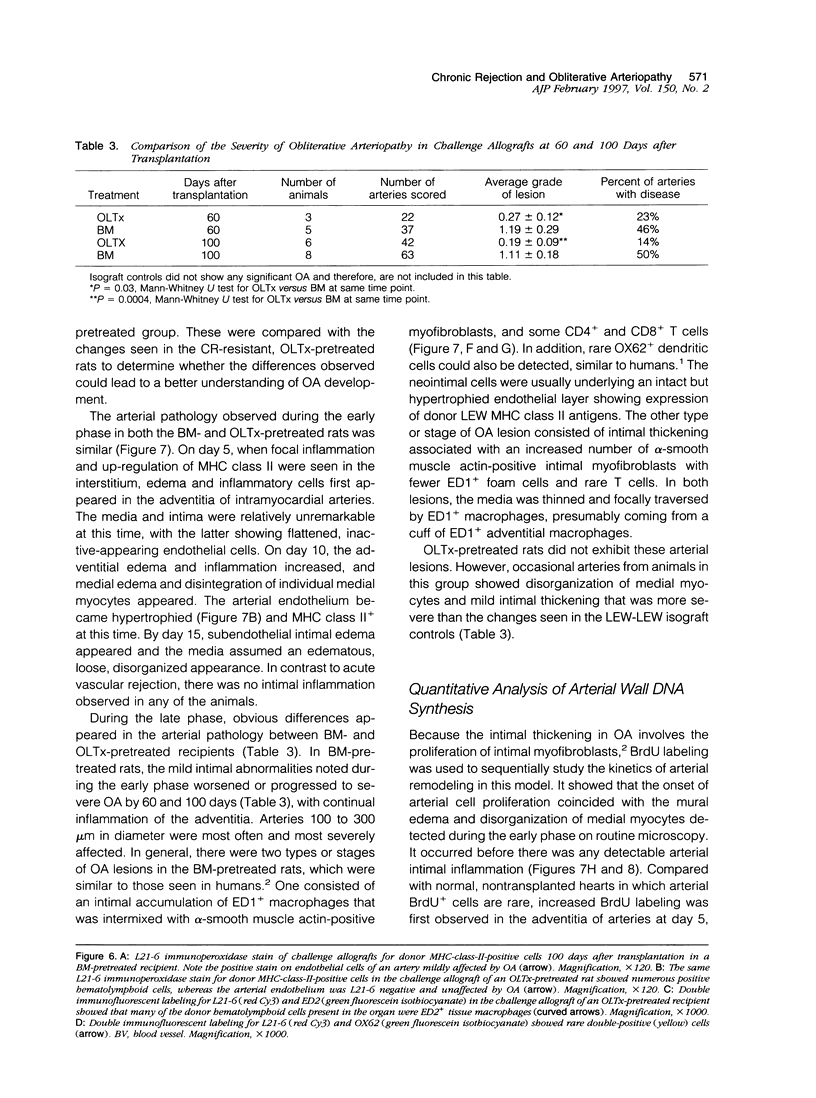

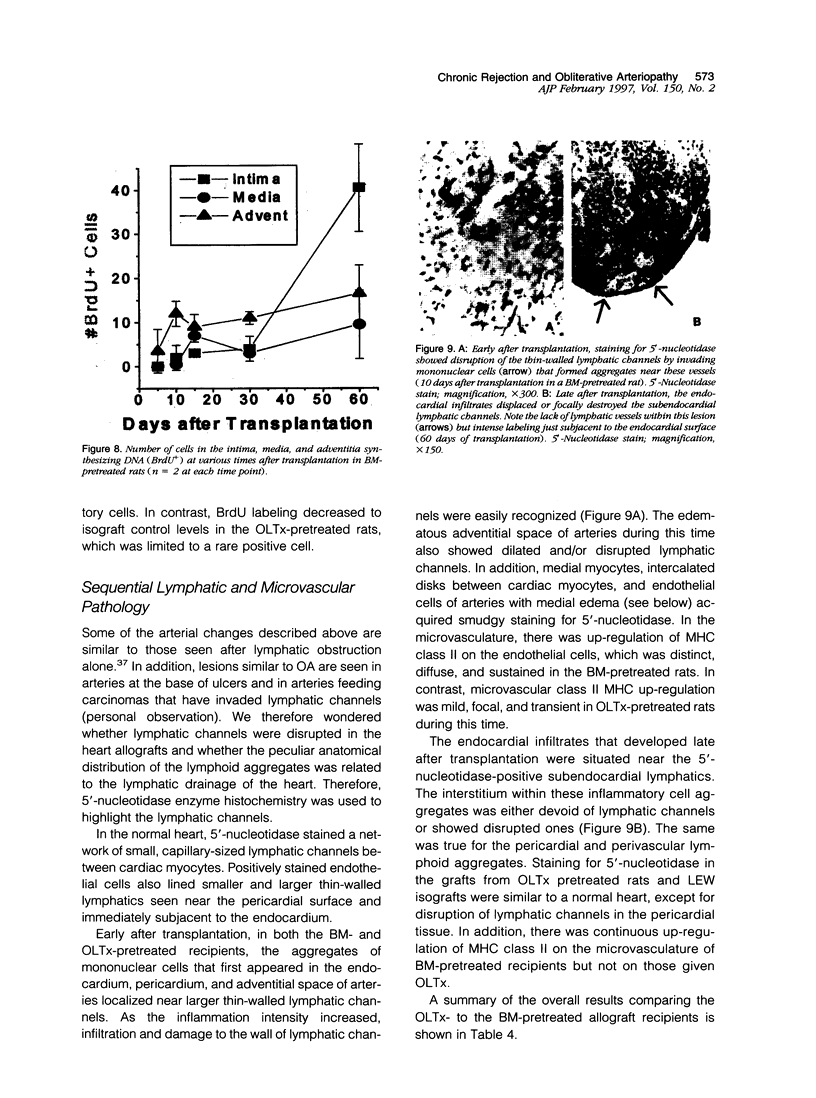

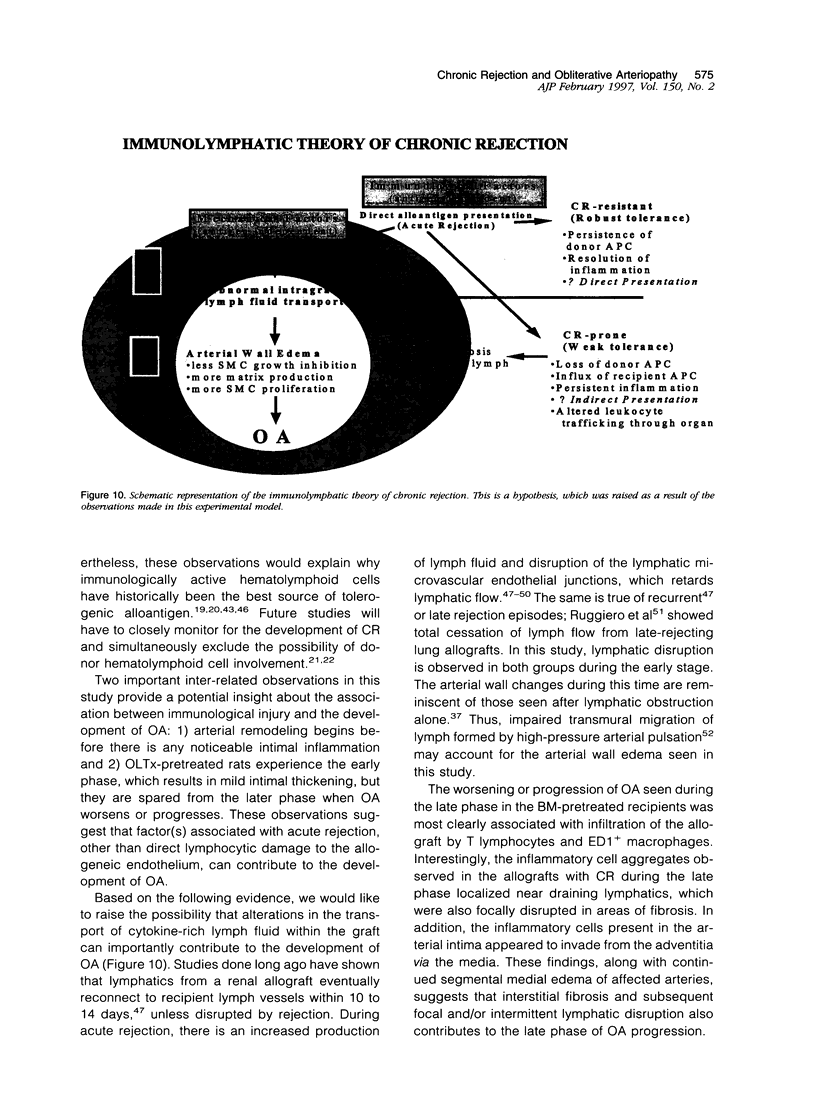



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Russell M. E., Hancock W. W., Sayegh M. H., Wyner L. R., Karnovsky M. J. Chronic rejection in experimental cardiac transplantation: studies in the Lewis-F344 model. Immunol Rev. 1993 Aug;134:5–19. doi: 10.1111/j.1600-065x.1993.tb00637.x. [DOI] [PubMed] [Google Scholar]
- Arnold B., Schönrich G., Hämmerling G. J. Multiple levels of peripheral tolerance. Immunol Today. 1993 Jan;14(1):12–14. doi: 10.1016/0167-5699(93)90317-E. [DOI] [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Bertolino P., Heath W. R., Hardy C. L., Morahan G., Miller J. F. Peripheral deletion of autoreactive CD8+ T cells in transgenic mice expressing H-2Kb in the liver. Eur J Immunol. 1995 Jul;25(7):1932–1942. doi: 10.1002/eji.1830250721. [DOI] [PubMed] [Google Scholar]
- Billingham M. E. Pathology and etiology of chronic rejection of the heart. Clin Transplant. 1994 Jun;8(3 Pt 2):289–292. [PubMed] [Google Scholar]
- Billingham M. E. The postsurgical heart. The pathology of cardiac transplantation. Am J Cardiovasc Pathol. 1988;1(3):319–334. [PubMed] [Google Scholar]
- Brenner C. A., Tam A. W., Nelson P. A., Engleman E. G., Suzuki N., Fry K. E., Larrick J. W. Message amplification phenotyping (MAPPing): a technique to simultaneously measure multiple mRNAs from small numbers of cells. Biotechniques. 1989 Nov-Dec;7(10):1096–1103. [PubMed] [Google Scholar]
- Bushell A., Pearson T. C., Morris P. J., Wood K. J. Donor-recipient microchimerism is not required for tolerance induction following recipient pretreatment with donor-specific transfusion and anti-CD4 antibody. Evidence of a clear role for short-term antigen persistence. Transplantation. 1995 May 27;59(10):1367–1371. doi: 10.1097/00007890-199505270-00001. [DOI] [PubMed] [Google Scholar]
- Chen-Woan M., Delaney C. P., Fournier V., Wakizaka Y., Murase N., Fung J., Starzl T. E., Demetris A. J. In vitro characterization of rat bone marrow-derived dendritic cells and their precursors. J Leukoc Biol. 1996 Feb;59(2):196–207. doi: 10.1002/jlb.59.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cockett A. T., Sakai A., Netto I. C. Kidney lymphatics: an important network in transplantation. Trans Am Assoc Genitourin Surg. 1973;65:73–76. [PubMed] [Google Scholar]
- Costanzo-Nordin M. R., Winters G. L., Fisher S. G., O'Sullivan J., Heroux A. L., Kao W., Mullen G. M., Johnson M. R. Endocardial infiltrates in the transplanted heart: clinical significance emerging from the analysis of 5026 endomyocardial biopsy specimens. J Heart Lung Transplant. 1993 Sep-Oct;12(5):741–747. [PubMed] [Google Scholar]
- Cramer D. V., Qian S. Q., Harnaha J., Chapman F. A., Estes L. W., Starzl T. E., Makowka L. Cardiac transplantation in the rat. I. The effect of histocompatibility differences on graft arteriosclerosis. Transplantation. 1989 Mar;47(3):414–419. doi: 10.1097/00007890-198903000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetris A. J., Murase N., Delaney C. P., Woan M., Fung J. J., Starzl T. E. The liver allograft, chronic (ductopenic) rejection, and microchimerism: what can they teach us? Transplant Proc. 1995 Feb;27(1):67–70. [PMC free article] [PubMed] [Google Scholar]
- Demetris A. J., Murase N., Starzl T. E. Donor dendritic cells after liver and heart allotransplantation under short-term immunosuppression. Lancet. 1992 Jun 27;339(8809):1610–1610. doi: 10.1016/0140-6736(92)91875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetris A. J., Qian S., Sun H., Fung J. J., Yagihashi A., Murase N., Iwaki Y., Gambrell B., Starzl T. E. Early events in liver allograft rejection. Delineation of sites of simultaneous intragraft and recipient lymphoid tissue sensitization. Am J Pathol. 1991 Mar;138(3):609–618. [PMC free article] [PubMed] [Google Scholar]
- Demetris A. J., Zerbe T., Banner B. Morphology of solid organ allograft arteriopathy: identification of proliferating intimal cell populations. Transplant Proc. 1989 Aug;21(4):3667–3669. [PubMed] [Google Scholar]
- Eliska O., Elisková M., Mirejovský P. Lymph vessels of the transplanted kidney. Nephron. 1986;44(2):136–141. doi: 10.1159/000184218. [DOI] [PubMed] [Google Scholar]
- Forbes R. D., Rowan R. A., Billingham M. E. Endocardial infiltrates in human heart transplants: a serial biopsy analysis comparing four immunosuppression protocols. Hum Pathol. 1990 Aug;21(8):850–855. doi: 10.1016/0046-8177(90)90055-a. [DOI] [PubMed] [Google Scholar]
- Häyry P., Isoniemi H., Yilmaz S., Mennander A., Lemström K., Räisänen-Sokolowski A., Koskinen P., Ustinov J., Lautenschlager I., Taskinen E. Chronic allograft rejection. Immunol Rev. 1993 Aug;134:33–81. doi: 10.1111/j.1600-065x.1993.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Ildstad S. T., Sachs D. H. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984 Jan 12;307(5947):168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- Joshi A., Masek M. A., Brown B. W., Jr, Weiss L. M., Billingham M. E. "Quilty" revisited: a 10-year perspective. Hum Pathol. 1995 May;26(5):547–557. doi: 10.1016/0046-8177(95)90252-x. [DOI] [PubMed] [Google Scholar]
- Kamada N., Calne R. Y. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979 Jul;28(1):47–50. [PubMed] [Google Scholar]
- Kato S., Yasunaga A., Uchida U. Enzyme-histochemical method for identification of lymphatic capillaries. Lymphology. 1991 Sep;24(3):125–129. [PubMed] [Google Scholar]
- Keenan R. J., Zeevi A., Iacono A. T., Spichty K. J., Cai J. Z., Yousem S. A., Ohori N. P., Paradis I. L., Kawai A., Griffith B. P. Efficacy of inhaled cyclosporine in lung transplant recipients with refractory rejection: correlation of intragraft cytokine gene expression with pulmonary function and histologic characteristics. Surgery. 1995 Aug;118(2):385–391. doi: 10.1016/s0039-6060(05)80349-6. [DOI] [PubMed] [Google Scholar]
- Kemnitz J., Cohnert T., Schäfers H. J., Helmke M., Wahlers T., Herrmann G., Schmidt R. M., Haverich A. A classification of cardiac allograft rejection. A modification of the classification by Billingham. Am J Surg Pathol. 1987 Jul;11(7):503–515. doi: 10.1097/00000478-198707000-00002. [DOI] [PubMed] [Google Scholar]
- Kemnitz J., Cremer J., Schaefers H. J., Restrepo-Specht I., Haverich A., Uysal A., Heublein B., Wirth S. Some aspects of changed histopathologic appearance of acute rejection in cardiac allografts after prophylactic application of OKT3. J Heart Lung Transplant. 1991 May-Jun;10(3):366–372. [PubMed] [Google Scholar]
- Kottke-Marchant K., Ratliff N. B. Endomyocardial lymphocytic infiltrates in cardiac transplant recipients. Incidence and characterization. Arch Pathol Lab Med. 1989 Jun;113(6):690–698. [PubMed] [Google Scholar]
- Lu L., Rudert W. A., Qian S., McCaslin D., Fu F., Rao A. S., Trucco M., Fung J. J., Starzl T. E., Thomson A. W. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J Exp Med. 1995 Aug 1;182(2):379–387. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIN J. M., PREHN R. T. Successful skin homografts after the administration of high dosage X radiation and homologous bone marrow. J Natl Cancer Inst. 1955 Feb;15(4):1023–1029. [PubMed] [Google Scholar]
- Maeda T., Eto M., Nishimura Y., Nomoto K., Kong Y. Y., Nomoto K. Role of peripheral hemopoietic chimerism in achieving donor-specific tolerance in adult mice. J Immunol. 1993 Feb 1;150(3):753–762. [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Mosnier J. F., Degott C., Marcellin P., Hénin D., Erlinger S., Benhamou J. P. The intraportal lymphoid nodule and its environment in chronic active hepatitis C: an immunohistochemical study. Hepatology. 1993 Mar;17(3):366–371. [PubMed] [Google Scholar]
- Murase N., Starzl T. E., Tanabe M., Fujisaki S., Miyazawa H., Ye Q., Delaney C. P., Fung J. J., Demetris A. J. Variable chimerism, graft-versus-host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to brown Norway rats. Transplantation. 1995 Jul 27;60(2):158–171. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N., Starzl T. E., Ye Q., Tsamandas A., Thomson A. W., Rao A. S., Demetris A. J. Multilineage hematopoietic reconstitution of supralethally irradiated rats by syngeneic whole organ transplantation. With oarticular reference to the liver. Transplantation. 1996 Jan 15;61(1):1–4. doi: 10.1097/00007890-199601150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málek P., Vrubel J., Kolc J. Lymphatic aspects of experimental and clinical renal transplantation. Bull Soc Int Chir. 1969 Jan-Feb;28(1):110–114. [PubMed] [Google Scholar]
- Málek P., Vrubel J. Lymphatic system and organ transplantation. Lymphology. 1968 Mar;1(1):4–22. [PubMed] [Google Scholar]
- Oguma S., Banner B., Zerbe T., Starzl T., Demetris A. J. Participation of dendritic cells in vascular lesions of chronic rejection of human allografts. Lancet. 1988 Oct 22;2(8617):933–936. doi: 10.1016/s0140-6736(88)92600-1. [DOI] [PubMed] [Google Scholar]
- Ono K., Lindsey E. S. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969 Feb;57(2):225–229. [PubMed] [Google Scholar]
- Owen R. D. IMMUNOGENETIC CONSEQUENCES OF VASCULAR ANASTOMOSES BETWEEN BOVINE TWINS. Science. 1945 Oct 19;102(2651):400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- Pardo-Mindán F. J., Lozano M. D., Contreras-Mejuto F., de Alava E. Pathology of heart transplant through endomyocardial biopsy. Semin Diagn Pathol. 1992 Aug;9(3):238–248. [PubMed] [Google Scholar]
- Paul L. C. Immunobiology of chronic renal transplant rejection. Blood Purif. 1995;13(3-4):206–218. doi: 10.1159/000170203. [DOI] [PubMed] [Google Scholar]
- Qian S., Demetris A. J., Murase N., Rao A. S., Fung J. J., Starzl T. E. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994 Apr;19(4):916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radio S. J., McManus B. M., Winters G. L., Kendall T. J., Wilson J. E., Costanzo-Nordin M. R., Ye Y. L. Preferential endocardial residence of B-cells in the "Quilty effect" of human heart allografts: immunohistochemical distinction from rejection. Mod Pathol. 1991 Sep;4(5):654–660. [PubMed] [Google Scholar]
- Reinsmoen N. L., Jackson A., McSherry C., Ninova D., Wiesner R. H., Kondo M., Krom R. A., Hertz M. I., Bolman R. M., 3rd, Matas A. J. Organ-specific patterns of donor antigen-specific hyporeactivity and peripheral blood allogeneic microchimerism in lung, kidney, and liver transplant recipients. Transplantation. 1995 Dec 27;60(12):1546–1554. doi: 10.1097/00007890-199560120-00029. [DOI] [PubMed] [Google Scholar]
- Ruggiero R., Fietsam R., Jr, Thomas G. A., Muz J., Farris R. H., Kowal T. A., Myles J. L., Stephenson L. W., Baciewicz F. A., Jr Detection of canine allograft lung rejection by pulmonary lymphoscintigraphy. J Thorac Cardiovasc Surg. 1994 Aug;108(2):253–258. [PubMed] [Google Scholar]
- Schmid-Schönbein G. W. Microlymphatics and lymph flow. Physiol Rev. 1990 Oct;70(4):987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Schönrich G., Alferink J., Klevenz A., Küblbeck G., Auphan N., Schmitt-Verhulst A. M., Hämmerling G. J., Arnold B. Tolerance induction as a multi-step process. Eur J Immunol. 1994 Feb;24(2):285–293. doi: 10.1002/eji.1830240202. [DOI] [PubMed] [Google Scholar]
- Shirwan H., Wang H. K., Barwari L., Makowka L., Cramer D. V. Pretransplant injection of allograft recipients with donor blood or lymphocytes permits allograft tolerance without the presence of persistent donor microchimerism. Transplantation. 1996 May 15;61(9):1382–1386. doi: 10.1097/00007890-199605150-00017. [DOI] [PubMed] [Google Scholar]
- Solti F., Jellinek H., Schneider F., Lengyel E., Bérczi V., Kékesi V. Lymphatic arteriopathy: damage to the wall of the canine femoral artery after lymphatic blockade. Lymphology. 1991 Jun;24(2):54–59. [PubMed] [Google Scholar]
- Starzl T. E., Demetris A. J., Murase N., Ildstad S., Ricordi C., Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992 Jun 27;339(8809):1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Demetris A. J. Transplantation milestones. Viewed with one- and two-way paradigms of tolerance. JAMA. 1995 Mar 15;273(11):876–879. doi: 10.1001/jama.273.11.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H., Toyoshima T., Fukao K., Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996 Feb;2(2):198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- Tullius S. G., Hancock W. W., Heemann U., Azuma H., Tilney N. L. Reversibility of chronic renal allograft rejection. Critical effect of time after transplantation suggests both host immune dependent and independent phases of progressive injury. Transplantation. 1994 Jul 15;58(1):93–99. [PubMed] [Google Scholar]
- Werner J. A., Schünke M., Tillmann B. Histochemical visualization of lymphatic capillaries in the rat: a comparison of methods demonstrated at the posterior pharyngeal surface. Arch Histol Jpn. 1987 Dec;50(5):505–514. doi: 10.1679/aohc.50.505. [DOI] [PubMed] [Google Scholar]
- Yagihashi A., Takahashi S., Murase N., Starzl T. E., Iwaki Y. A monoclonal antibody (L21-6) recognizing an invariant chain expressed on the cell surface in rats with the exception of the BN (RT1n): a study of tissue and strain distributions. Transplant Proc. 1995 Apr;27(2):1519–1521. [PMC free article] [PubMed] [Google Scholar]
- Yousem S. A., Paradis I. L., Dauber J. H., Zeevi A., Duquesnoy R. J., Dal Col R., Armitage J., Hardesty R. L., Griffith B. P. Pulmonary arteriosclerosis in long-term human heart-lung transplant recipients. Transplantation. 1989 Mar;47(3):564–569. [PubMed] [Google Scholar]
- van Saase J. L., van der Woude F. J., Thorogood J., Hollander A. A., van Es L. A., Weening J. J., van Bockel J. H., Bruijn J. A. The relation between acute vascular and interstitial renal allograft rejection and subsequent chronic rejection. Transplantation. 1995 May 15;59(9):1280–1285. [PubMed] [Google Scholar]