Abstract
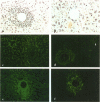
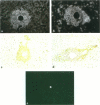
Synchronized liver granulomas were induced by injecting Sepharose beads to which SEA soluble egg antigen (SEA) or the concanavalin A binding fraction of SEA had been coupled into a mesenteric vein in naive, single-sex (35 days) and bisexually (28 days) Schistosoma mansoni-infected and Plasmodium berghei-immunized mice. Stereological analysis revealed that peak granuloma formation was already reached 8 days after injection in single-sex infected mice compared with 16 days in naive animals. No difference in granuloma formation between naive and P. berghei-immunized animals and between unisexually and bisexually S. mansoni-infected mice was observed. This suggests that the positive immunomodulatory effect on the granulomogenesis is worm specific and not likely to be due to arousal of the immune system by unrelated factors, nor is it influenced by the gender or degree of maturation of female worms. At all stages in time, the concanavalin A binding-fraction-induced granulomas reached only 65 to 70% of the volume of SEA-induced granulomas. Immunophenotyping of extracellular matrix proteins around deposited heads revealed that fibronectin was the dominant extracellular matrix protein and that also type I and IV collagen and laminin were deposited. Temporal analysis of the expression of the adhesion molecules ICAM-1, LFA-1, VLA-4, and VLA-6 was performed. Morphological evidence is presented for the role of adhesion molecules in the initiation and maintenance of hepatic granuloma formation. The chemokine monocyte chemoattractant protein-1 was expressed in the granuloma and in hepatic artery branches. From these data, it is concluded that adult S. mansoni worms positively modulate schistosomal hepatic granuloma formation in vivo. Adhesion molecules and chemokines play important roles in schistosomal granuloma formation.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade Z. A., Grimaud J. A. Evolution of the schistosomal hepatic lesions in mice after curative chemotherapy. Am J Pathol. 1986 Jul;124(1):59–65. [PMC free article] [PubMed] [Google Scholar]
- Araújo M. I., de Jesus A. R., Bacellar O., Sabin E., Pearce E., Carvalho E. M. Evidence of a T helper type 2 activation in human schistosomiasis. Eur J Immunol. 1996 Jun;26(6):1399–1403. doi: 10.1002/eji.1830260633. [DOI] [PubMed] [Google Scholar]
- BIGUET J., CAPRON A., TRAN VAN KY P. [Schistosoma mansoni antigens. I. Electrophoresis and immunoelectrophoresis study. Characterization of specific antigens]. Ann Inst Pasteur (Paris) 1962 Nov;103:763–777. [PubMed] [Google Scholar]
- BROWNE H. G., THOMAS J. I. A method for isolating pure, viable schistosome eggs from host tissues. J Parasitol. 1963 Jun;49:371–374. [PubMed] [Google Scholar]
- Bogers J. J., Nibbeling H. A., Deelder A. M., van Marck E. A. Immunohistochemical and ultrastructural localization of Schistosoma mansoni soluble egg antigens processed by the infected host. Parasitology. 1996 Jun;112(Pt 6):537–543. doi: 10.1017/s0031182000066117. [DOI] [PubMed] [Google Scholar]
- Boros D. L. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989 Jul;2(3):250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A., Biguet J., Vernes A., Afchain D. Structure antigénique des helminthes. Aspects immunologiques des relations hote-parasite. Pathol Biol. 1968 Feb;16(3):121–138. [PubMed] [Google Scholar]
- Chensue S. W., Warmington K. S., Lukacs N. W., Lincoln P. M., Burdick M. D., Strieter R. M., Kunkel S. L. Monocyte chemotactic protein expression during schistosome egg granuloma formation. Sequence of production, localization, contribution, and regulation. Am J Pathol. 1995 Jan;146(1):130–138. [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Warmington K., Ruth J., Lincoln P., Kuo M. C., Kunkel S. L. Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am J Pathol. 1994 Nov;145(5):1105–1113. [PMC free article] [PubMed] [Google Scholar]
- Claassen E. Alkaline phosphatase-fast red fluorescence. Rediscovery of a useful label? J Immunol Methods. 1991 May 17;139(1):149–151. doi: 10.1016/0022-1759(91)90362-j. [DOI] [PubMed] [Google Scholar]
- Deelder A. M., Kornelis D., Van Marck E. A., Eveleigh P. C., Van Egmond J. G. Schistosoma mansoni: characterization of two circulating polysaccharide antigens and the immunological response to these antigens in mouse, hamster, and human infections. Exp Parasitol. 1980 Aug;50(1):16–32. doi: 10.1016/0014-4894(80)90004-1. [DOI] [PubMed] [Google Scholar]
- Deelder A. M., Snoijink J. J., Ploem J. S. Experimental optimization of the DASS system for immunodiagnosis of some helminth infections. Ann N Y Acad Sci. 1975 Jun 30;254:119–136. doi: 10.1111/j.1749-6632.1975.tb29162.x. [DOI] [PubMed] [Google Scholar]
- Doughty B. L., Phillips S. M. Delayed hypersensitivity granuloma formation around Schistosoma mansoni eggs in vitro. I. Definition of the model. J Immunol. 1982 Jan;128(1):30–36. [PubMed] [Google Scholar]
- Edungbola L. D., Schiller E. L. Histopathology of hepatic and pulmonary granulomata experimentally induced with eggs of Schistosoma mansoni. J Parasitol. 1979 Apr;65(2):253–261. [PubMed] [Google Scholar]
- Eltoum I. A., Wynn T. A., Poindexter R. W., Finkelman F. D., Lewis F. A., Sher A., Cheever A. W. Suppressive effect of interleukin-4 neutralization differs for granulomas around Schistosoma mansoni eggs injected into mice compared with those around eggs laid in infected mice. Infect Immun. 1995 Jul;63(7):2532–2536. doi: 10.1128/iai.63.7.2532-2536.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaud J. A., Boros D. L., Takiya C., Mathew R. C., Emonard H. Collagen isotypes, laminin, and fibronectin in granulomas of the liver and intestines of Schistosoma mansoni-infected mice. Am J Trop Med Hyg. 1987 Sep;37(2):335–344. doi: 10.4269/ajtmh.1987.37.335. [DOI] [PubMed] [Google Scholar]
- Grzych J. M., Pearce E., Cheever A., Caulada Z. A., Caspar P., Heiny S., Lewis F., Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991 Feb 15;146(4):1322–1327. [PubMed] [Google Scholar]
- Hamburger J., Pelley R. P., Warren K. S. Schistoma mansoni soluble egg antigens: determination of the stage and species specificity of their serologic reactivity by radioimmunoassay. J Immunol. 1976 Nov;117(5 Pt 1):1561–1566. [PubMed] [Google Scholar]
- Harrison D. J., Carter C. E., Colley D. G. Immunoaffinity purification of Schistosoma mansoni soluble egg antigens. J Immunol. 1979 Jun;122(6):2210–2217. [PubMed] [Google Scholar]
- Hirsch C., Goes A. M. Characterization of fractionated Schistosoma mansoni soluble adult worm antigens that elicit human cell proliferation and granuloma formation in vitro. Parasitology. 1996 Jun;112(Pt 6):529–535. doi: 10.1017/s0031182000066105. [DOI] [PubMed] [Google Scholar]
- Kresina T. F., He Q., Degli Esposti S., Zern M. A. Gene expression of transforming growth factor beta 1 and extracellular matrix proteins in murine Schistosoma mansoni infection. Gastroenterology. 1994 Sep;107(3):773–780. doi: 10.1016/0016-5085(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Langley J. G., Boros D. L. T-lymphocyte responsiveness in murine schistosomiasis mansoni is dependent upon the adhesion molecules intercellular adhesion molecule-1, lymphocyte function-associated antigen-1, and very late antigen-4. Infect Immun. 1995 Oct;63(10):3980–3986. doi: 10.1128/iai.63.10.3980-3986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Lymphokine regulation of granuloma formation in murine schistosomiasis mansoni. Clin Immunol Immunopathol. 1993 Jul;68(1):57–63. doi: 10.1006/clin.1993.1095. [DOI] [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Splenic and granuloma T-lymphocyte responses to fractionated soluble egg antigens of Schistosoma mansoni-infected mice. Infect Immun. 1991 Mar;59(3):941–948. doi: 10.1128/iai.59.3.941-948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Utilization of fractionated soluble egg antigens reveals selectively modulated granulomatous and lymphokine responses during murine schistosomiasis mansoni. Infect Immun. 1992 Aug;60(8):3209–3216. doi: 10.1128/iai.60.8.3209-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs N. W., Chensue S. W., Strieter R. M., Warmington K., Kunkel S. L. Inflammatory granuloma formation is mediated by TNF-alpha-inducible intercellular adhesion molecule-1. J Immunol. 1994 Jun 15;152(12):5883–5889. [PubMed] [Google Scholar]
- Lukacs N. W., Kunkel S. L., Strieter R. M., Warmington K., Chensue S. W. The role of macrophage inflammatory protein 1 alpha in Schistosoma mansoni egg-induced granulomatous inflammation. J Exp Med. 1993 Jun 1;177(6):1551–1559. doi: 10.1084/jem.177.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustigman S., Mahmoud A. A., Hamburger J. Glycopeptides in soluble egg antigen of Schistosoma mansoni: isolation, characterization, and elucidation of their immunochemical and immunopathological relation to the major egg glycoprotein (MEG). J Immunol. 1985 Mar;134(3):1961–1967. [PubMed] [Google Scholar]
- Nash T. E. Factors that modulate clearance and ultimate fate of a specific schistosome antigen (GASP) in schistosome infections. J Immunol. 1982 Apr;128(4):1608–1613. [PubMed] [Google Scholar]
- Pelley R. P., Pelley R. J., Hamburger J., Peters P. A., Warren K. S. Schistosoma mansoni soluble egg antigens. I. Identification and purification of three major antigens, and the employment of radioimmunoassay for their further characterization. J Immunol. 1976 Nov;117(5 Pt 1):1553–1560. [PubMed] [Google Scholar]
- Ritter D. M., McKerrow J. H. Intercellular adhesion molecule 1 is the major adhesion molecule expressed during schistosome granuloma formation. Infect Immun. 1996 Nov;64(11):4706–4713. doi: 10.1128/iai.64.11.4706-4713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama M. M., Aronstein W. S., Weiss J. B., Strand M. Monoclonal antibody identification of protein antigens in the liver of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1984 Jul;33(4):608–620. doi: 10.4269/ajtmh.1984.33.608. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Stocker S., Van Marck E. A., Deelder A. M., Kestens L., Gigase P. L., Grimaud J. A. Hepatic schistosomal fibrosis: ultrastructural study of experimentally induced periparticular reaction. Contrib Microbiol Immunol. 1983;7:260–266. [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993 Jan 7;361(6407):79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Van Marck E. A., Kestens L., Stocker S., Grimaud J. A., Gigase P. L., Deelder A. M. Fibrosis around schistosomal egg antigen-coated beads in the liver of mice. Contrib Microbiol Immunol. 1983;7:251–259. [PubMed] [Google Scholar]
- Van Marck E. Presence of the circulating polysaccharide antigen in the liver of mice infected with Schistosoma mansoni. Ann Soc Belg Med Trop. 1975;55(4):373–377. [PubMed] [Google Scholar]
- Weiss J. B., Aronstein W. S., Strand M. Schistosoma mansoni: stimulation of artificial granuloma formation in vivo by carbohydrate determinants. Exp Parasitol. 1987 Oct;64(2):228–236. doi: 10.1016/0014-4894(87)90147-0. [DOI] [PubMed] [Google Scholar]
- Williams M. E., Montenegro S., Domingues A. L., Wynn T. A., Teixeira K., Mahanty S., Coutinho A., Sher A. Leukocytes of patients with Schistosoma mansoni respond with a Th2 pattern of cytokine production to mitogen or egg antigens but with a Th0 pattern to worm antigens. J Infect Dis. 1994 Oct;170(4):946–954. doi: 10.1093/infdis/170.4.946. [DOI] [PubMed] [Google Scholar]