Abstract
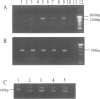
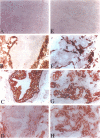
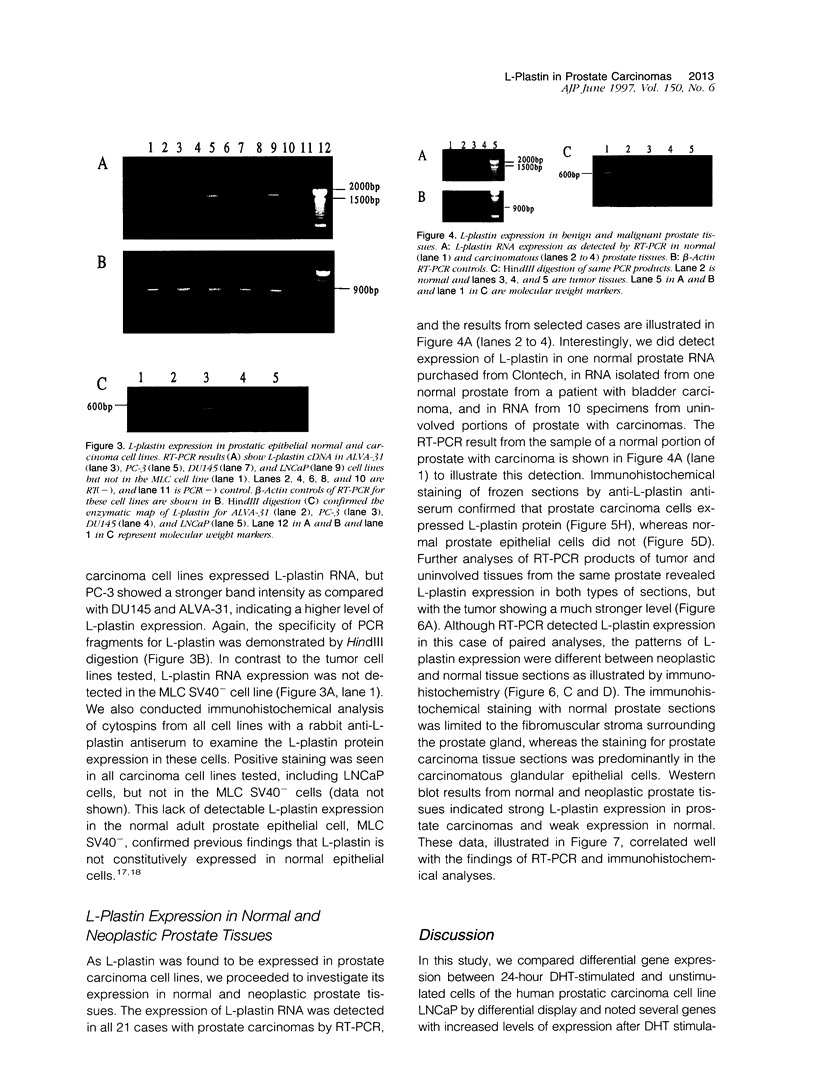
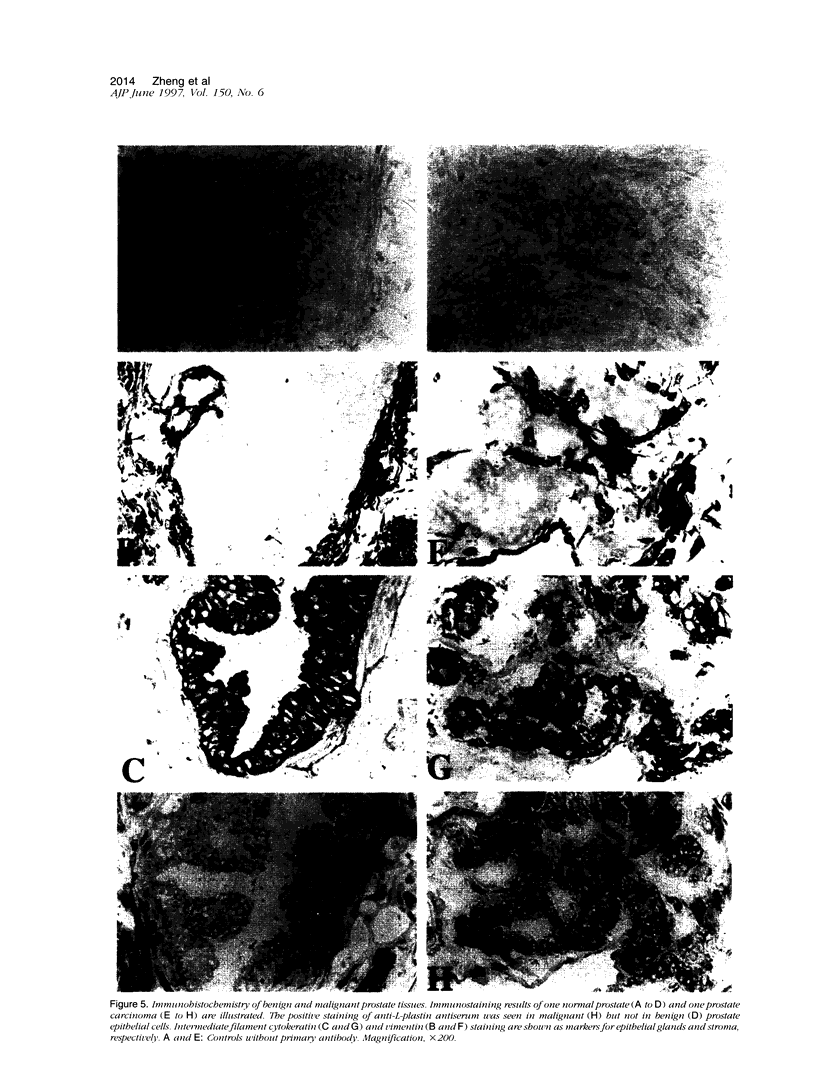
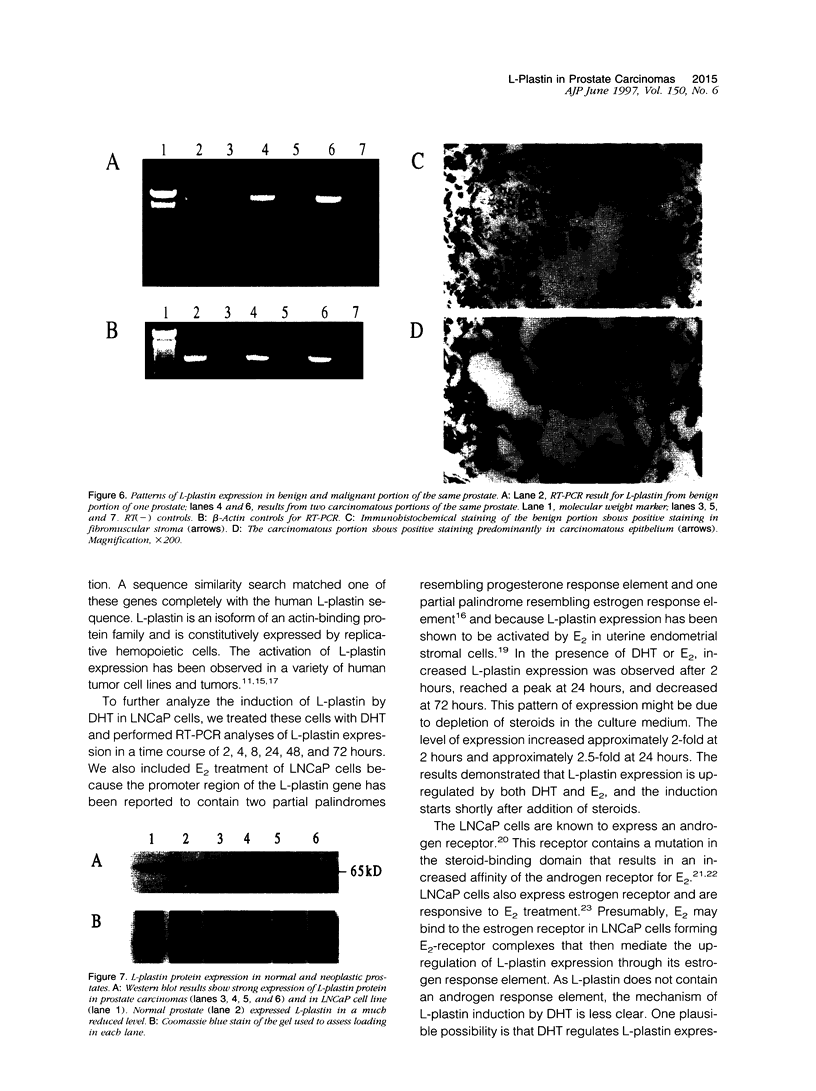
Application of differential display to the comparison of androgen-stimulated and unstimulated human prostate carcinoma cell line LNCaP identified androgen induction of the L-plastin gene, which encodes an actin-binding protein isoform. Further investigation demonstrated that L-plastin expression in LNCaP cells is up-regulated by both dihydrotestosterone and estradiol. This induction of expression is detected as early as 2 hours after addition of steroids to the cell culture. L-plastin expression is also detected in other prostate carcinoma cell lines by reverse transcriptase polymerase chain reaction and immunohistochemistry but not in the single normal adult prostate epithelial cell line that is available to us. Analysis of multiple primary prostatic tumor tissues as well as normal and tumor tissues of the same prostate gland showed that tumor tissues exhibit a higher level of expression as compared with the normal tissues. Immunohistochemical study using anti-L-plastin antiserum on normal and carcinomatous prostate tissues showed a very striking difference in the staining patterns. Positive staining was seen in the fibromuscular stroma in normal prostates but not in the glandular epithelial cells. In contrast, strong staining was seen predominantly within the carcinomatous glandular epithelial cells. Taken together, these results suggest that the expression of L-plastin in prostatic epithelial cells is linked to the malignant state and that, once expressed in carcinomas, its expression is regulated by steroid hormone receptors.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arpin M., Friederich E., Algrain M., Vernel F., Louvard D. Functional differences between L- and T-plastin isoforms. J Cell Biol. 1994 Dec;127(6 Pt 2):1995–2008. doi: 10.1083/jcb.127.6.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T., Montgomery S. Cancer statistics, 1994. CA Cancer J Clin. 1994 Jan-Feb;44(1):7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- Carter H. B., Coffey D. S. The prostate: an increasing medical problem. Prostate. 1990;16(1):39–48. doi: 10.1002/pros.2990160105. [DOI] [PubMed] [Google Scholar]
- Castagnetta L. A., Miceli M. D., Sorci C. M., Pfeffer U., Farruggio R., Oliveri G., Calabrò M., Carruba G. Growth of LNCaP human prostate cancer cells is stimulated by estradiol via its own receptor. Endocrinology. 1995 May;136(5):2309–2319. doi: 10.1210/endo.136.5.7536668. [DOI] [PubMed] [Google Scholar]
- Catalona W. J. Management of cancer of the prostate. N Engl J Med. 1994 Oct 13;331(15):996–1004. doi: 10.1056/NEJM199410133311507. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988 Apr 15;240(4850):324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Gittes R. F. Carcinoma of the prostate. N Engl J Med. 1991 Jan 24;324(4):236–245. doi: 10.1056/NEJM199101243240406. [DOI] [PubMed] [Google Scholar]
- Goldstein D., Djeu J., Latter G., Burbeck S., Leavitt J. Abundant synthesis of the transformation-induced protein of neoplastic human fibroblasts, plastin, in normal lymphocytes. Cancer Res. 1985 Nov;45(11 Pt 2):5643–5647. [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Chu T. M., Wajsman Z. L., Friedman M., Papsidero L., Kim U., Chai L. S., Kakati S., Arya S. K. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Kawinski E., Karr J. P., Rosenthal H., Chu T. M., Mirand E. A., Murphy G. P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983 Apr;43(4):1809–1818. [PubMed] [Google Scholar]
- Isaacs W. B., Bova G. S., Morton R. A., Bussemakers M. J., Brooks J. D., Ewing C. M. Molecular biology of prostate cancer. Semin Oncol. 1994 Oct;21(5):514–521. [PubMed] [Google Scholar]
- Leavitt J., Chen Z. P., Lockwood C. J., Schatz F. Regulation of synthesis of the transformation-induced protein, leukocyte plastin, by ovarian steroid hormones. Cancer Res. 1994 Jul 1;54(13):3447–3454. [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Aebersold R. H., Kent S. B., Varma M., Leavitt J. Molecular cloning and characterization of plastin, a human leukocyte protein expressed in transformed human fibroblasts. Mol Cell Biol. 1988 Nov;8(11):4659–4668. doi: 10.1128/mcb.8.11.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Aebersold R. H., Leavitt J. Correction of the N-terminal sequences of the human plastin isoforms by using anchored polymerase chain reaction: identification of a potential calcium-binding domain. Mol Cell Biol. 1990 Apr;10(4):1818–1821. doi: 10.1128/mcb.10.4.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Chen Z. P., Park T., Ghosh K., Leavitt J. Characterization of the human L-plastin gene promoter in normal and neoplastic cells. J Biol Chem. 1993 Feb 5;268(4):2793–2801. [PubMed] [Google Scholar]
- Lin C. S., Park T., Chen Z. P., Leavitt J. Human plastin genes. Comparative gene structure, chromosome location, and differential expression in normal and neoplastic cells. J Biol Chem. 1993 Feb 5;268(4):2781–2792. [PubMed] [Google Scholar]
- Lin C. S., Shen W., Chen Z. P., Tu Y. H., Matsudaira P. Identification of I-plastin, a human fimbrin isoform expressed in intestine and kidney. Mol Cell Biol. 1994 Apr;14(4):2457–2467. doi: 10.1128/mcb.14.4.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Modular organization of actin crosslinking proteins. Trends Biochem Sci. 1991 Mar;16(3):87–92. doi: 10.1016/0968-0004(91)90039-x. [DOI] [PubMed] [Google Scholar]
- Messier J. M., Shaw L. M., Chafel M., Matsudaira P., Mercurio A. M. Fimbrin localized to an insoluble cytoskeletal fraction is constitutively phosphorylated on its headpiece domain in adherent macrophages. Cell Motil Cytoskeleton. 1993;25(3):223–233. doi: 10.1002/cm.970250303. [DOI] [PubMed] [Google Scholar]
- Miller G. J., Stapleton G. E., Hedlund T. E., Moffat K. A. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1alpha,25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res. 1995 Sep;1(9):997–1003. [PubMed] [Google Scholar]
- Namba Y., Ito M., Zu Y., Shigesada K., Maruyama K. Human T cell L-plastin bundles actin filaments in a calcium-dependent manner. J Biochem. 1992 Oct;112(4):503–507. doi: 10.1093/oxfordjournals.jbchem.a123929. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Derancourt J. Purification and further characterization of macrophage 70-kDa protein, a calcium-regulated, actin-binding protein identical to L-plastin. Biochemistry. 1993 Apr 6;32(13):3448–3455. doi: 10.1021/bi00064a031. [DOI] [PubMed] [Google Scholar]
- Park T., Chen Z. P., Leavitt J. Activation of the leukocyte plastin gene occurs in most human cancer cells. Cancer Res. 1994 Apr 1;54(7):1775–1781. [PubMed] [Google Scholar]
- Parker M. G., White R., Williams J. G. Cloning and characterization of androgen-dependent mRNA from rat ventral prostate. J Biol Chem. 1980 Jul 25;255(14):6996–7001. [PubMed] [Google Scholar]
- Peeters B. L., Mous J. M., Rombauts W. A., Heyns W. J. Androgen-induced messenger RNA in rat ventral prostate. Translation, partial purification, and preliminary characterization of the mRNAs encoding the components of prostatic binding protein. J Biol Chem. 1980 Jul 25;255(14):7017–7023. [PubMed] [Google Scholar]
- Perry J. E., Tindall D. J. Androgens regulate the expression of proliferating cell nuclear antigen posttranscriptionally in the human prostate cancer cell line, LNCaP. Cancer Res. 1996 Apr 1;56(7):1539–1544. [PubMed] [Google Scholar]
- Tan J. A., Marschke K. B., Ho K. C., Perry S. T., Wilson E. M., French F. S. Response elements of the androgen-regulated C3 gene. J Biol Chem. 1992 Mar 5;267(7):4456–4466. [PubMed] [Google Scholar]
- Veldscholte J., Ris-Stalpers C., Kuiper G. G., Jenster G., Berrevoets C., Claassen E., van Rooij H. C., Trapman J., Brinkmann A. O., Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990 Dec 14;173(2):534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- Veldscholte J., Voorhorst-Ogink M. M., Bolt-de Vries J., van Rooij H. C., Trapman J., Mulder E. Unusual specificity of the androgen receptor in the human prostate tumor cell line LNCaP: high affinity for progestagenic and estrogenic steroids. Biochim Biophys Acta. 1990 Apr 9;1052(1):187–194. doi: 10.1016/0167-4889(90)90075-o. [DOI] [PubMed] [Google Scholar]
- Wilding G. The importance of steroid hormones in prostate cancer. Cancer Surv. 1992;14:113–130. [PubMed] [Google Scholar]
- Yoshimura I., Wu J. M., Chen Y., Ng C., Mallouh C., Backer J. M., Mendola C. E., Tazaki H. Effects of 5 alpha-dihydrotestosterone (DHT) on the transcription of nm23 and c-myc genes in human prostatic LNCaP cells. Biochem Biophys Res Commun. 1995 Mar 17;208(2):603–609. doi: 10.1006/bbrc.1995.1381. [DOI] [PubMed] [Google Scholar]
- Young C. Y., Andrews P. E., Montgomery B. T., Tindall D. J. Tissue-specific and hormonal regulation of human prostate-specific glandular kallikrein. Biochemistry. 1992 Jan 28;31(3):818–824. doi: 10.1021/bi00118a026. [DOI] [PubMed] [Google Scholar]
- Zu Y. L., Shigesada K., Nishida E., Kubota I., Kohno M., Hanaoka M., Namba Y. 65-kilodalton protein phosphorylated by interleukin 2 stimulation bears two putative actin-binding sites and two calcium-binding sites. Biochemistry. 1990 Sep 11;29(36):8319–8324. doi: 10.1021/bi00488a017. [DOI] [PubMed] [Google Scholar]
- de Arruda M. V., Watson S., Lin C. S., Leavitt J., Matsudaira P. Fimbrin is a homologue of the cytoplasmic phosphoprotein plastin and has domains homologous with calmodulin and actin gelation proteins. J Cell Biol. 1990 Sep;111(3):1069–1079. doi: 10.1083/jcb.111.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]